22260-51-1
| 中文名 | 甲磺酸溴隐亭 |
|---|---|
| 英文名 | bromocriptine methanesulfonate |
| 中文别名 |
甲黄酸溴麦角环肽
2-溴-alpha-麦角环肽甲磺酸盐 |
| 英文别名 |
(5'α)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxoergotaman methanesulfonate (salt)
Bromocriptine mesilate (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide methanesulfonate (salt) CB-154 mesylate acide méthanesulfonique - (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-méthyléthyl)-5-(2-méthylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-méthyl-4,6,6a,7, Apo-Bromocriptine 2-Bromine-a-ergocryptine Methanesulfonate Parlodel 2-Bromo α-Ergocryptine Mesylate (5'α)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-3',6',18-trioxo-2'-(propan-2-yl)ergotaman methanesulfonate (1:1) hydroindolo[4,3-fg]chinolin-9-carboxamid(1:1) acide méthanesulfonique - (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-méthyléthyl)-5-(2-méthylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-méthyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoléine-9-carboxamide (1:1) Methansulfonsäure--(6aR,9R)-5-brom-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]chinolin-9-carboxamid(1:1) EINECS 244-881-1 2-Bromo-12'-hydroxy-5'a-isobutyl-2'-isopropylergotaman-3',6',18-trione Monomethanesulphonate (5'α)-2-Bromo-12'-hydroxy-5'-isobutyl-2'-isopropyl-3',6',18-trioxoergotaman methanesulfonate (1:1) Ergotaman, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxo-, (5'α)-, methanesulfonate (1:1) (salt) (6aR,9R)-5-bromo-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg Methansulfonsäure--(6aR,9R)-5-brom-N-[(2R,5S,10aS,10bS)-10b-hydroxy-2-(1-methylethyl)-5-(2-methylpropyl)-3,6-dioxooctahydro-8H-[1,3]oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl]-7-methyl-4,6,6a,7,8,9-hexa MFCD00069218 8,9-hexahydroindolo[4,3-fg]quinoléine-9-carboxamide (1:1) Bromocriptine (mesylate) |
| 描述 | Bromocriptine mesylate 是一种有效的多巴胺 D2/D3 受体激动剂,结合多巴胺 D2 受体,pKi 为 8.05±0.2。 |
|---|---|
| 相关类别 | |
| 靶点 |
pKi: 8.05±0.2 (dopamine D2 receptor)[1] |
| 体外研究 | Bromocriptine刺激[35S]-GTPγS与CHO细胞中表达的D2多巴胺受体结合,pEC50为8.15±0.05 [1]。 Bromocriptine也是脑一氧化氮合酶的强抑制剂。麦角生物碱Bromocriptine(BKT)被发现作为纯化的神经元型一氧化氮合酶(NOS)的强抑制剂(IC50 = 10±2μM),而它对诱导型巨噬细胞NOS(IC50>100μM)的活性很差[2] 。发现溴隐亭抑制至少一种人细胞色素P450酶的活性。 Bromocriptine是一种有效的CYP3A4抑制剂,计算出的相互作用IC50值为1.69μM[3]。 |
| 体内研究 | 在强迫游泳试验(FST)和尾部悬吊试验(TST)中,在小鼠组中给予甲磺酸布洛霉素(2mg/kg,ip)7天。与对照相比,Bromocriptine组显示出显着的抗不动作用。当在最后一次7天MPE处理后30分钟给予溴隐亭并进行FST时,与单独的MPE处理相比,该多巴胺能激动剂产生MPE(200mg/kg,口服)的抗不动作用的显着和剂量依赖性增强。与对照相比,Bromocriptine治疗组显示出不动时间的显着减少。用MPE(100和200mg/kg,po)预处理7天后给予溴隐亭,与单独的MPE治疗相比,显示出MPE的抗不动作用的显着和剂量依赖性增强[4]。与假手术(注射盐水的大鼠)相比,脑室内给予溴隐亭显着降低静态机械异常性疼痛(SMA)评分,并且其效果持续30分钟。与假手术相比,腹膜内给予溴隐亭诱导CCI-IoN组疼痛评分显着,剂量依赖性(0.1 mg和1 mg/kg)降低,其效果持续6 h。最高剂量诱导评分降低最高(P <0.01)。 Bromocriptine效应持续20分钟。与假手术相比,腹膜内施用溴隐亭诱导CCI-IoN + 6-OHDA损伤组中SMA评分的显着剂量依赖性降低。它的效果持续6小时[5]。 |
| 激酶实验 | 进行[35S]-GTPγS结合测定。将细胞膜(25±75μg)在含有0.1mM二硫苏糖醇(DTT)和1μMGDP的缓冲液B和体积为0.9mL的药物中于30℃温育30分钟。当加入[35S]-GTPγS(50±150pM,最终浓度)(在100uL缓冲液B中)以引发反应时,该预温育确保测试的激动剂处于平衡状态。除非另有说明,否则将测定混合物再温育20分钟。通过快速过滤终止测定,并如上文对放射配体结合测定所述测定结合的放射活性。 [35S]-GTPγS的总结合小于添加[1]的20%。 |
| 动物实验 | 小鼠[4]使用任一性别的瑞士小鼠(20-25g)(总共150只)。 Bromocriptine mesylate用作多巴胺受体(D2)激动剂。氟哌啶醇在蒸馏水中稀释,蒸馏水用于注射载体。将甲磺酸布洛霉素溶于一滴冰醋酸中,并在蒸馏水中定容。将丙咪嗪溶于0.9%生理盐水中。在强迫游泳试验(FST)和尾部悬浮试验(TST)中,在小鼠组中施用氟哌啶醇(0.1mg / kg,ip)和布洛霉素甲磺酸盐(2mg / kg,ip)7天。作为标准的丙咪嗪(10mg / kg,口服)在阳性对照组中施用7天。大鼠[5]使用成年雄性Sprague-Dawley大鼠(N = 112,275-325g)。注射6-OHDA两周后,使用面罩将动物短暂(<3分钟)用2%氟烷麻醉,并接受脑池内给药Bromocriptine(溶于5μL载体中的7μg/ kg)或单独的载体(5μL) 0.9%盐水)。对于ip注射,我们使用Bromocriptine(1mg / kg)和SKF81297(3mg / kg溶于0.9%盐水中)浓度。在恢复期(<2分钟)后,将大鼠置于观察区域中40分钟,由盲实验者进行测试。 |
| 参考文献 |
| 沸点 | 891.3ºC at 760 mmHg |
|---|---|
| 分子式 | C33H44BrN5O8S |
| 分子量 | 750.700 |
| 闪点 | 492.8ºC |
| 精确质量 | 749.209412 |
| PSA | 180.96000 |
| LogP | 3.98220 |
| 外观性状 | 白色固体 |
| 蒸汽压 | 0mmHg at 25°C |
| 储存条件 | 2-8℃ |
| 稳定性 | 常温常压下稳定 避免接触 强氧化剂 |
| 水溶解性 | H2O: 0.8 mg/mL |
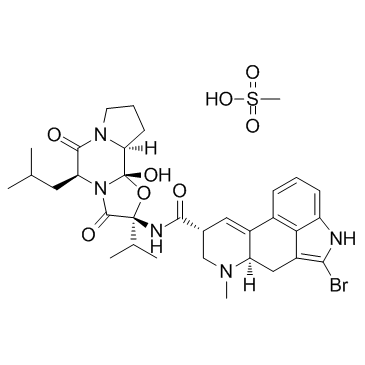
| 分子结构 | |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:4 3.氢键受体数量:9 4.可旋转化学键数量:5 5.互变异构体数量:8 6.拓扑分子极性表面积181 7.重原子数量:48 8.表面电荷:0 9.复杂度:1320 10.同位素原子数量:0 11.确定原子立构中心数量:6 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:2 |
| 更多 | 1.性状:白色固体 2.密度(g/mL,25/4℃):未确定 3.相对蒸汽密度(g/mL,空气=1):未确定 4.熔点(ºC):未确定 5.沸点(ºC,常压):未确定 6.沸点(ºC,5.2kPa):未确定 7.折射率:未确定 8.闪点(ºC):未确定 9.比旋光度(º):未确定 10.自燃点或引燃温度(ºC):未确定 11.蒸气压(kPa,25ºC):未确定 12.饱和蒸气压(kPa,60ºC):未确定 13.燃烧热(KJ/mol):未确定 14.临界温度(ºC):未确定 15.临界压力(KPa):未确定 16.油水(辛醇/水)分配系数的对数值:未确定 17.爆炸上限(%,V/V):未确定 18.爆炸下限(%,V/V):未确定 19.溶解性:溶于水 |
|
毒理学数据: 生态学数据: CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |


GHS07, GHS09 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H302-H410 |
| 警示性声明 | P301 + P312 + P330 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| 危害码 (欧洲) | Xn: Harmful; |
| 风险声明 (欧洲) | R20/21/22 |
| 安全声明 (欧洲) | S22-S24/25 |
| 危险品运输编码 | UN 3077 9 / PGIII |
| WGK德国 | 3 |
| RTECS号 | KE1595000 |