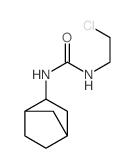
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YS5075000
-
CHEMICAL NAME :
-
Urea, 1-(2-chloroethyl)-1-nitroso-3-(2-norbornyl)-
-
CAS REGISTRY NUMBER :
-
13909-13-2
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
6
-
MOLECULAR FORMULA :
-
C10-H16-Cl-N3-O2
-
MOLECULAR WEIGHT :
-
245.74
-
WISWESSER LINE NOTATION :
-
L55 ATJ CMVNNO&2G
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
83 mg/kg
-
TOXIC EFFECTS :
-
Liver - jaundice, other or unclassified Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NCIMR* Progress Report Submitted to the National Cancer Institute by Mason Research Institute. (Worcester, MA) Volume(issue)/page/year: -,372,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
56 mg/kg
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NCIMR* Progress Report Submitted to the National Cancer Institute by Mason Research Institute. (Worcester, MA) Volume(issue)/page/year: -,372,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
54760 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NCISP* National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. (Bethesda, MD 20205) Volume(issue)/page/year: JAN1986 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
18750 ug/kg/15D-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
NCIMR* Progress Report Submitted to the National Cancer Institute by Mason Research Institute. (Worcester, MA) Volume(issue)/page/year: -,372,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
15 mg/kg/5W-I
-
TOXIC EFFECTS :
-
Liver - change in gall bladder structure or function Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
NCIMR* Progress Report Submitted to the National Cancer Institute by Mason Research Institute. (Worcester, MA) Volume(issue)/page/year: -,372,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
35 mg/kg/15D-I
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NCIMR* Progress Report Submitted to the National Cancer Institute by Mason Research Institute. (Worcester, MA) Volume(issue)/page/year: -,372,1968
|

![双环[2.2.1]-2-庚胺结构式](https://image.chemsrc.com/caspic/383/822-98-0.png)