Josamycin propionate
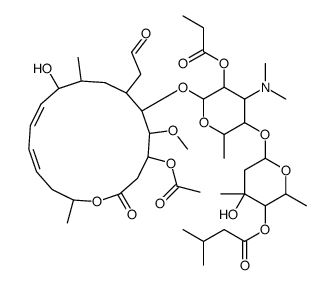
Josamycin propionate结构式

|
常用名 | Josamycin propionate | 英文名 | Josamycin propionate |
|---|---|---|---|---|
| CAS号 | 31674-19-8 | 分子量 | 884.05800 | |
| 密度 | 1.19g/cm3 | 沸点 | 946.1ºC at 760 mmHg | |
| 分子式 | C45H73NO16 | 熔点 | N/A | |
| MSDS | N/A | 闪点 | 526ºC |
Josamycin propionate用途Josamycin propionate is a macrolide antibiotic. It is synthesized from strains of Streptomyces narbonensis var. josamyceticus var. nova |
| 英文名 | [6-[6-[[(11Z,13E)-4-acetyloxy-10-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-7-(2-oxoethyl)-1-oxacyclohexadeca-11,13-dien-6-yl]oxy]-4-(dimethylamino)-2-methyl-5-propanoyloxyoxan-3-yl]oxy-4-hydroxy-2,4-dimethyloxan-3-yl] 3-methylbutanoate |
|---|---|
| 英文别名 | 更多 |
| 密度 | 1.19g/cm3 |
|---|---|
| 沸点 | 946.1ºC at 760 mmHg |
| 分子式 | C45H73NO16 |
| 分子量 | 884.05800 |
| 闪点 | 526ºC |
| 精确质量 | 883.49300 |
| PSA | 212.12000 |
| LogP | 3.97430 |
|
Application of liquid chromatography-ion trap mass spectrometry to the characterization of the 16-membered ring macrolide josamycin propionate.
J. Mass Spectrom. 39(4) , 437-46, (2004) Coupled liquid chromatography and ion trap mass spectrometry (LC/MS) was used for the characterization of the semi-synthetic 16-membered ring macrolide josamycin propionate. On-line identification of ... |
|
|
A rapid and sensitive high-pressure liquid chromatographic method for monitoring josamycin levels in plasma.
Int. J. Clin. Pharmacol. Res. 4(3) , 195-9, (1984) In this study a new high-pressure liquid chromatographic method for monitoring plasma levels of josamycin is described. Josamycin propionate was used as the internal standard. After being extracted fr... |
|
|
Clinical multicentre trial with josamycin propionate in paediatric patients.
Int. J. Clin. Pharmacol. Res. 4(3) , 201-7, (1984) Josamycin propionate, a tasteless josamycin derivative suitable for the preparation of paediatric oral suspension, was employed in a large, multicentre clinical study aimed at evaluating the effective... |
| 2'-O-Methyl-5-methylcytidine |
| Leucomycin V 3-acetate 4B-(3-methylbutanoate) 2A-propanoate |
| 2'-O-Propionyl-LM-A4 |


