Androstan-16-one,3-hydroxy-, (3b,5a)-
更新时间:2024-01-13 13:01:06
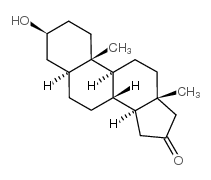
Androstan-16-one,3-hydroxy-, (3b,5a)-结构式

|
常用名 | Androstan-16-one,3-hydroxy-, (3b,5a)- | 英文名 | Androstan-16-one,3-hydroxy-, (3b,5a)- |
|---|---|---|---|---|
| CAS号 | 571-51-7 | 分子量 | 290.44000 | |
| 密度 | 1.085g/cm3 | 沸点 | 413.1ºC at 760mmHg | |
| 分子式 | C19H30O2 | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | 176.4ºC |
| 英文名 | 5alpha-androstan-3beta-ol-16-one |
|---|---|
| 英文别名 | 更多 |
| 密度 | 1.085g/cm3 |
|---|---|
| 沸点 | 413.1ºC at 760mmHg |
| 分子式 | C19H30O2 |
| 分子量 | 290.44000 |
| 闪点 | 176.4ºC |
| 精确质量 | 290.22500 |
| PSA | 37.30000 |
| LogP | 3.95910 |
| 折射率 | 1.536 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
|
Hydrogen transfer between C19 steroids during oxidoreduction at C-17 in vivo.
Biochim. Biophys. Acta 711(1) , 149-58, (1982)
|
|
|
Effects of ethanol metabolism on oxidoreduction and intermolecular hydrogen transfer at C-17 in steroid 3-sulphates in vivo.
Biochim. Biophys. Acta 711(1) , 159-65, (1982) Steroid sulphates were infused intravenously in female rats, and metabolites were isolated from bile. Infused 3 beta-hydroxy-5 alpha-androstan-17-one 3-sulphate was excreted together with 5 alpha-andr... |
| 5A-ANDROSTAN-3B-OL-16-ONE |
| 3-hydroxyandrostan-16-one |
| 3BETA-HYDROXY-5ALPHA-ANDROSTAN-16-ONE |
