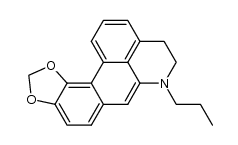
(-)-MDO-NPA HCl
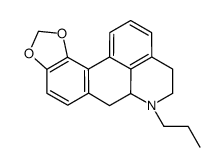
(-)-MDO-NPA HCl结构式

|
常用名 | (-)-MDO-NPA HCl | 英文名 | (-)-MDO-NPA HCl |
|---|---|---|---|---|
| CAS号 | 81264-57-5 | 分子量 | 307.38600 | |
| 密度 | 1.209g/cm3 | 沸点 | 456ºC at 760 mmHg | |
| 分子式 | C20H21NO2 | 熔点 | 250-252ºC | |
| MSDS | 美版 | 闪点 | 165.2ºC |
(-)-MDO-NPA HCl用途(-)-MDO-NPA HCl is an orally effective long-acting agent active at central dopamine receptors, and analogous aporphines. |
| 英文名 | (6aR)-7-Propyl-6a,7,8,9-tetrahydro-6H-benzo[de][1,3]benzodioxolo[ 4,5-g]quinoline |
|---|---|
| 英文别名 | 更多 |
| 密度 | 1.209g/cm3 |
|---|---|
| 沸点 | 456ºC at 760 mmHg |
| 熔点 | 250-252ºC |
| 分子式 | C20H21NO2 |
| 分子量 | 307.38600 |
| 闪点 | 165.2ºC |
| 精确质量 | 307.15700 |
| PSA | 21.70000 |
| LogP | 3.88550 |
| 折射率 | 1.622 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
|
~92% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Journal of Organic Chemistry, 1981 , vol. 46, # 13 p. 2830 - 2831 |
|
~% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Journal of Organic Chemistry, 1981 , vol. 46, # 13 p. 2830 - 2831 |
|
~% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 947 - 949 |
|
~% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 947 - 949 |
|
~% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 947 - 949 |
|
~% 
(-)-MDO-NPA HCl 81264-57-5 |
| 文献:Ram, Vishnu J.; Neumeyer, John L. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 947 - 949 |
|
Behavioral effects of (-)10,11-methylenedioxy-N-n-propylnoraporphine, an orally effective long-acting agent active at central dopamine receptors, and analogous aporphines.
Neuropharmacology 21(10) , 953-61, (1982) Substituted and unsubstituted 10,11-methylenedioxy derivatives of apomorphine (APO) or its N-propyl congener (NPA) were synthesized and evaluated for their ability to alter motor activity or to induce... |
|
|
Dopaminergic stimulation of oxytocin concentrations in the plasma of male and female monkeys by apomorphine and a D2 receptor agonist.
J. Clin. Endocrinol. Metab. 75(3) , 855-60, (1992) Administration of the dopamine receptor agonist apomorphine causes a dose-dependent increase in plasma oxytocin concentrations and dose-specific behavioral changes in rodents. To investigate whether d... |
|
|
Tissue levels of N-n-propylnorapomorphine after treatment with (-)10,11-methylenedioxy-N-n-propylnoraporphine, an orally long-acting prodrug active at central dopamine receptors.
Neuropharmacology 21(12) , 1311-6, (1982) High performance liquid chromatography with electrochemical detection was used to assay N-n-propylnorapomorphine (NPA) and other aporphines. Pretreatment of rats with (-)10,11-methylenedioxy-N-n-propy... |
| (R)-MDO-NPA |
| (S)-Propane-1,2-diamine dihydrochloride |
| R-(-)-1,2-propanediamine dihydrochloride |
| (S)-1,2-diaminopropane |
| (S)-diaminopropane dihydrochloride |
| R(-)-10,11-Methylenedioxy-N-(n-propyl)noraporphine |
| (S)-1,2-propanediamine*2HCl |
| (S)-1,2-Diaminopropane 2HCl |
| S-(-)-1,2-diaminopropane dihydrochloride |
| (S)-1,2-Propanediamine dihydrochloride |

![(R)-11-((1-phenyl-1H-tetrazol-5-yl)oxy)-7-propyl-6a,7,8,9-tetrahydro-6H-[1,3]dioxolo[4',5':5,6]benzo[1,2-g]benzo[de]quinoline hydrochloride结构式](https://image.chemsrc.com/caspic/016/77630-06-9.png)
![[11C]-(-)-N-n-Propylnorapomorphine结构式](https://image.chemsrc.com/caspic/246/18426-20-5.png)
![(6aR)-5,6,6a,7-Tetrahydro-4H-dibenzo[de,g]quinoline-10,11-diol hy drobromide (1:1)结构式](https://image.chemsrc.com/caspic/013/115017-61-3.png)
![(6aR)-6-propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-6-ium-10,11-diol,chloride结构式](https://image.chemsrc.com/caspic/321/20382-71-2.png)

