73671-86-0
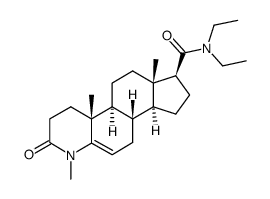
| Name | (7S,11aR)-N,N-Diethyl-1,4a,6a-trimethyl-2-oxohexadecahydro-1H-ind eno[5,4-f]quinoline-7-carboxamide |
|---|
| Description | DMAA is an indirect sympathomimetic amine. DMAA constricts blood vessels and raises blood pressure. DMAA can be used for neurological and cardiovascular disease research[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Rodricks JV, et al. DMAA as a dietary ingredient. JAMA Intern Med. 2013 Apr 8;173(7):594-5. |
| Density | 1.051g/cm3 |
|---|---|
| Boiling Point | 528.3ºC at 760 mmHg |
| Molecular Formula | C24H40N2O2 |
| Molecular Weight | 388.59 |
| Flash Point | 209.8ºC |
| Exact Mass | 388.30900 |
| PSA | 40.62000 |
| LogP | 4.27230 |
| Index of Refraction | 1.519 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~96% 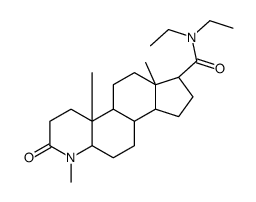
73671-86-0 |
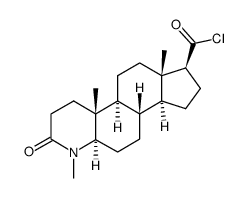
| Literature: Rasmusson, Gary H.; Reynolds, Glenn F.; Utne, Torleif; Jobson, Ronald B.; Primka, Raymond L.; et al. Journal of Medicinal Chemistry, 1984 , vol. 27, # 12 p. 1690 - 1701 |
|
~% 
73671-86-0 |
| Literature: Merck and Co., Inc. Patent: US4220775 A1, 1980 ; |
|
~% 
73671-86-0 |
| Literature: Rasmusson, Gary H.; Reynolds, Glenn F.; Utne, Torleif; Jobson, Ronald B.; Primka, Raymond L.; et al. Journal of Medicinal Chemistry, 1984 , vol. 27, # 12 p. 1690 - 1701 |
|
~% 
73671-86-0 |
| Literature: Rasmusson, Gary H.; Reynolds, Glenn F.; Utne, Torleif; Jobson, Ronald B.; Primka, Raymond L.; et al. Journal of Medicinal Chemistry, 1984 , vol. 27, # 12 p. 1690 - 1701 |
|
~% 
73671-86-0 |
| Literature: Rasmusson, Gary H.; Reynolds, Glenn F.; Utne, Torleif; Jobson, Ronald B.; Primka, Raymond L.; et al. Journal of Medicinal Chemistry, 1984 , vol. 27, # 12 p. 1690 - 1701 |