150148-81-5
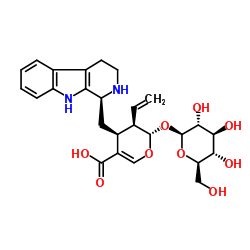
| Name | (2S,3R,4S)-2-(β-D-Glucopyranosyloxy)-4-[(1S)-2,3,4,9-tetrahydro-1H-β-carbolin-1-ylmethyl]-3-vinyl-3,4-dihydro-2H-pyran-5-carboxylic acid |
|---|---|
| Synonyms |
(2S,3R,4S)-2-(β-D-Glucopyranosyloxy)-4-[(1S)-2,3,4,9-tetrahydro-1H-β-carbolin-1-ylmethyl]-3-vinyl-3,4-dihydro-2H-pyran-5-carboxylic acid
(2S,3R,4S)-3-ethenyl-2-(β-D-glucopyranosyloxy)-4-[(1S)-2,3,4,9-tetrahydro-1H-β-carbolin-1-ylmethyl]-3,4-dihydro-2H-pyran-5-carboxylic acid 2H-Pyran-5-carboxylic acid, 3-ethenyl-2-(β-D-glucopyranosyloxy)-3,4-dihydro-4-[[(1S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl]methyl]-, (2S,3R,4S)- |
| Description | Strictosidinic acid, an orally active glycoside indole monoterpene alkaloid isolated from Psychotria myriantha leaves, inhibits precursor enzymes of 5-HT biosynthesis and reduces the 5-HT levels. Strictosidinic acid has peripheral analgesic and antipyretic activities in mice[1][2]. |
|---|---|
| Related Catalog | |
| Target |
5-HT Receptor |
| In Vivo | Strictosidinic acid (20 μg/μl; intra-hippocampal injection) causes a significance of 83.5% reduction in 5-HT levels. Strictosidinic acid (10 mg/kg; i.p.) causes a 63.4% reduction in 5-HT levels and a 67.4% reduction in DOPAC values in male Wistar rats, weighing 200-250 g[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 792.8±60.0 °C at 760 mmHg |
| Molecular Formula | C26H32N2O9 |
| Molecular Weight | 516.540 |
| Flash Point | 433.3±32.9 °C |
| Exact Mass | 516.210754 |
| LogP | -0.23 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | 2-8℃ |
| Hazard Codes | Xi |
|---|