1049741-03-8
| Name | TNF-α Inhibitor |
|---|---|
| Synonyms |
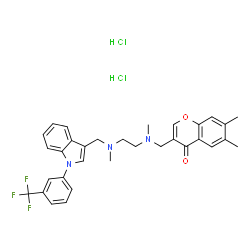
4H-1-Benzopyran-4-one, 6,7-dimethyl-3-[[methyl[2-[methyl[[1-[3-(trifluoromethyl)phenyl]-1H-indol-3-yl]methyl]amino]ethyl]amino]methyl]-, hydrochloride (1:2)
SPD00000304 MFCD09265259 6,7-Dimethyl-3-[(methyl{2-[methyl({1-[3-(trifluoromethyl)phenyl]-1H-indol-3-yl}methyl)amino]ethyl}amino)methyl]-4H-chromen-4-one dihydrochloride SPD304 6,7-Dimethyl-3-[[methyl[2-[methyl[[1-[3-(trifluoromethyl)phenyl]-1H-indol-3-yl]methyl]amino]ethyl]amino]methyl]-(4H-1-Benzopyran-4-one dihydrochloride |
| Description | SPD304 dihydrochloride is a selective TNF-α inhibitor, which promotes dissociation of TNF trimers and therefore blocks the interaction of TNF and its receptor. SPD304 has an IC50 of 22 µM for inhibiting in vitro TNF receptor 1 (TNFR1) binding to TNF-α[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 22 µM (TNFα)[1]. |
| In Vitro | SPD304 (2 μM) significantly rescues the survivability of aHSCs, reduces the production of lipid hydroxides, and increased intracellular GSH. The co-treatment of GA (75 μM) and SPD304 (2 μM), down-regulate TRADD almost 2-fold (w/o inhibitor vs. w/ inhibitor) and p−RIP3 1.4−fold compared to GA alone, and promotes caspase 8 activation[4]. |
| In Vivo | SPD304 cannot be used in vivo due to its high toxicity[3]. |
| References |
[1]. Molly M. He, et al. Small-Molecule Inhibition of TNF-α. Science 11 Nov 2005. |
| Molecular Formula | C32H34Cl2F3N3O2 |
|---|---|
| Molecular Weight | 620.532 |
| Exact Mass | 619.197998 |
| Appearance | white Solid |
| Storage condition | Store at -20° C |
| Water Solubility | Soluble in DMSO (10 mg/ml). |
| Hazard Codes | Xi |
|---|