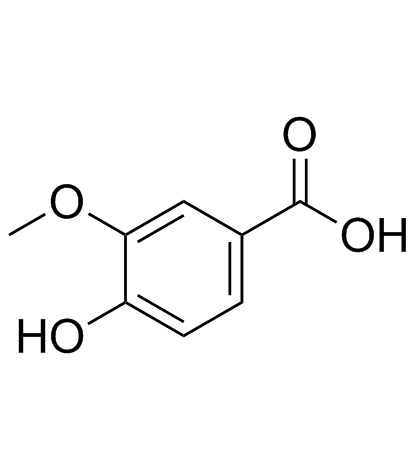
36341-25-0
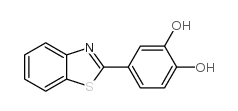
| Name | 4-(3H-1,3-benzothiazol-2-ylidene)-2-methoxycyclohexa-2,5-dien-1-one |
|---|---|
| Synonyms |
2-(4-hydroxy-3-methoxyphenyl)benzo[d]thiazole
2-(3-methoxy-4-hydroxyphenyl)benzothiazole 4-(benzo[d]thiazol-2-yl)-2-methoxyphenol 4-(1,3-benzothiazol-2-yl)-2-methoxyphenol Phenol,4-(2-benzothiazolyl)-2-methoxy 2-(4-HYDROXY-3-METHOXYPHENYL)BENZOTHIAZOLE 4-benzothiazol-2-yl-2-methoxyphenol YL-109 |
| Description | YL-109 is a novel anticancer agent which has ability to inhibit breast cancer cell growth and invasiveness in vitro and in vivo.IC50 value: 85.7 nM(MCF-7 cells proliferation) [1]Target: AhR signaling activcatorin vitro: YL-109 strongly inhibited cell proliferation of MCF-7 cells in a dose-dependent manner (IC50= 85.8 nM). Surprisingly, YL-109 had an anti-proliferative effect in a dose-dependent manner (IC50 = 4.02 μM) on MDA-MB-231 cells. YL-109 repressed the sphere-forming ability and the expression of stem cell markers in MDA-MB-231 mammosphere cultures. YL-109 increased the expression of carboxyl terminus of Hsp70-interacting protein (CHIP), which suppresses tumorigenic and metastatic potential of breast cancer cells by inhibiting the oncogenic pathway. YL-109 induced CHIP transcription because of the recruitment of the aryl hydrocarbon receptor (AhR) to upstream of CHIP gene in MDA-MB-231 cells. Consistently, the antitumor effects of YL-109 were depressed by CHIP or AhRknockdown in MDA-MB-231 cells [1].in vivo: Mice treated with vehicle showed significantly enlarged tumors, whereas mice treated with YL-109 showed attenuated tumor growth using MCF-7 cells. Interestingly, YL-109 also suppressed tumor growth in mice injected with MDA-MB-231 cells. Compared with the vehicle control, YL-109 significantly reduced lung metastasis [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.327g/cm3 |
|---|---|
| Boiling Point | 446.448ºC at 760 mmHg |
| Molecular Formula | C14H11NO2S |
| Molecular Weight | 257.30800 |
| Flash Point | 223.804ºC |
| Exact Mass | 257.05100 |
| PSA | 70.59000 |
| LogP | 3.67750 |
| Index of Refraction | 1.685 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2934200090 |
|
~94% 
36341-25-0 |
| Literature: Das, Sudipto; Samanta, Suvendu; Maji, Swarup Kumar; Samanta, Partha Kumar; Dutta, Amit Kumar; Srivastava, Divesh N.; Adhikary, Bibhutosh; Biswas, Papu Tetrahedron Letters, 2013 , vol. 54, # 9 p. 1090 - 1096 |
|
~85% 
36341-25-0 |
| Literature: Ma, Dawei; Xue, Peng; Jiang, Yongwen; Xie, Siwei; Zhang, Xiaojing; Dong, Jinhua Angewandte Chemie, International Edition, 2009 , vol. 48, # 23 p. 4222 - 4225 |
|
~68% 
36341-25-0 |
| Literature: Hyvl, Jakub; Srogl, Jiri European Journal of Organic Chemistry, 2010 , # 15 p. 2849 - 2851 |
|
~63% 
36341-25-0 |
| Literature: Liu, Saiwen; Chen, Ru; Guo, Xiangyu; Yang, Huiqiong; Deng, Guojun; Li, Chao-Jun Green Chemistry, 2012 , vol. 14, # 6 p. 1577 - 1580 |
|
~% 
36341-25-0 |
| Literature: Bogert; Stull Journal of the American Chemical Society, 1925 , vol. 47, p. 3078 Journal of the Chemical Society, 1926 , vol. 48, p. 250 Full Text View citing articles Show Details Claasz Chemische Berichte, 1916 , vol. 49, p. 1145 |
|
~% 
36341-25-0 |
| Literature: Bogert; Stull Journal of the American Chemical Society, 1925 , vol. 47, p. 3078 Journal of the Chemical Society, 1926 , vol. 48, p. 250 |
|
~% 
36341-25-0 |
| Literature: Rudolph, Joachim; Chen, Libing; Majumdar, Dyuti; Bullock, William H.; Burns, Michael; Claus, Thomas; Dela Cruz, Fernando E.; Daly, Michelle; Ehrgott, Frederick J.; Johnson, Jeffrey S.; Livingston, James N.; Schoenleber, Robert W.; Shapiro, Jeffrey; Yang, Ling; Tsutsumi, Manami; Ma, Xin Journal of Medicinal Chemistry, 2007 , vol. 50, # 5 p. 984 - 1000 |
| HS Code | 2934200090 |
|---|---|
| Summary | 2934200090. other compounds containing in the structure a benzothiazole ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |