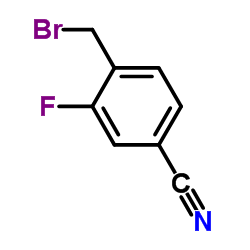
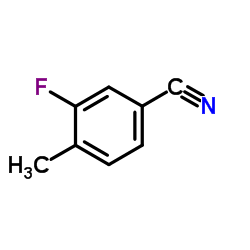
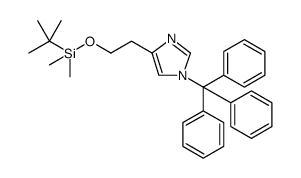
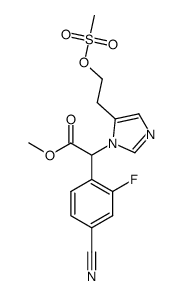
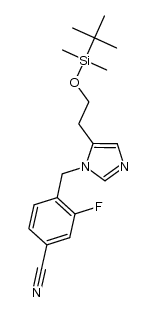
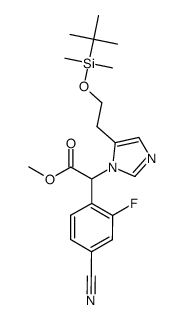
928134-65-0
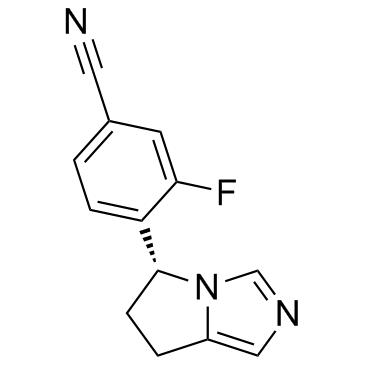
| Name | 4-[(5R)-6,7-Dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzo nitrile |
|---|---|
| Synonyms |
osilodrostat
4-(quinolin-3-ylmethyl-amino)-benzenesulfonamide Novartis LCI699 (R)-4-(6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl)-3-fluorobenzonitrile UNII-5YL4IQ1078 4-[(5R)-6,7-Dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile Benzonitrile, 4-[(5R)-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluoro- 3-<N-(4'-Amidosulfonylphenyl)aminomethyl>quinoline 4-((R)-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl)-3-fluoro-benzonitrile |
| Description | Osilodrostat (LCI699) is a potent inhibitor of human 11β-hydroxylase and aldosterone synthase with IC50 values of 2.5 and 0.7 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.5 nM (human 11β-hydroxylase), 0.7 nM (aldosterone synthase)[1] |
| In Vivo | Osilodrostat and pasireotide monotherapies are associated with significant changes in the histology and mean weights of the pituitary and adrenal glands, liver, and ovary/oviduct. Osilodrostat alone is associated with adrenocortical hypertrophy and hepatocellular hypertrophy. In combination, osilodrostat/pasireotide does not exacerbate any target organ changes and ameliorated the liver and adrenal gland changes observed with monotherapy. Cmax and AUC0–24h of osilodrostat and pasireotide increase in an approximately dose-proportional manner[1]. Osilodrostat treatment reduces urinary free cortisol in patients with Cushing's disease; 78.9% has normal urinary free cortisol at week 22. Treatment with osilodrostat is generally well tolerated[2]. |
| Animal Admin | Rats: Sixty male and 60 female rats are randomized into single-sex groups to receive daily doses of pasireotide (0.3 mg/kg/day, subcutaneously), osilodrostat (20 mg/kg/day, orally), osilodrostat/pasireotide in combination (low dose, 1.5/0.03 mg/kg/day; mid-dose, 5/0.1 mg/kg/day; or high dose, 20/0.3 mg/kg/day), or vehicle for 13 weeks[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 433.8±45.0 °C at 760 mmHg |
| Molecular Formula | C13H10FN3 |
| Molecular Weight | 227.237 |
| Flash Point | 216.2±28.7 °C |
| Exact Mass | 227.085876 |
| PSA | 41.61000 |
| LogP | 1.13 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | -20℃ |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
|
~% 
928134-65-0 |
| Literature: Meredith, Erik L.; Ksander, Gary; Monovich, Lauren G.; Papillon, Julien P. N.; Liu, Qian; Miranda, Karl; Morris, Patrick; Rao, Chang; Burgis, Robin; Capparelli, Michael; Hu, Qi-Ying; Singh, Alok; Rigel, Dean F.; Jeng, Arco Y.; Beil, Michael; Fu, Fumin; Hu, Chii-Whei; Lasala, Daniel ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 12 p. 1203 - 1207 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |