341-88-8
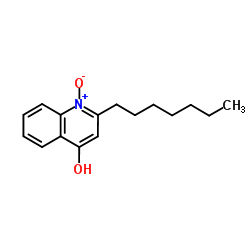
| Name | 2-heptyl-4-hydroxyquinoline N-oxide |
|---|---|
| Synonyms |
HOQNO
2-Heptylquinolin-4-ol 1-oxide 2 heptyl 4 hydroxyquinoline 1 oxide 4-Quinolinol,2-heptyl-,1-oxide 2-heptyl-1-hydroxyquinolin-4-one 2-Heptyl-4-quinolinol 1-oxide HQNO 4-Quinolinol, 2-heptyl-, 1-oxide 2-heptyl-4-hydroxyquinoline n-oxide |
| Description | HQNO, secreted by P. aeruginosa, is a potent electron transport chain inhibitor with a Kd of 64 nM for complex III[1]. HQNO is a potent inhibitor of mitochondrial NDH-2 in many species[2]. |
|---|---|
| Related Catalog | |
| Target |
NDH-2[2] |
| In Vitro | HQNO is a potent inhibitor of mitochondrial type II NADH:quinone oxidoreductase (NDH-2) in many species, including Yarrowia lipolytica, S. cerevisiae, Gluconobacter oxydans, T. gondii, P. falciparum, and S. aureus. HQNO targets the Q-site of NDH-2[2]. HQNO concentrations are varied from 0 to 100 μM and 0 to 300 μM for wild-type (WT) and I379E C. thermarum NDH-2 variant, respectively to determine IC50 values. WT NDH-2 has an IC50 value of 10.5±1.3 μM HQNO in the presence of 400 μM Menadione (MD). In the presence of 50 μM MD, the IC50 value for HQNO decreases slightly to 7.3±1.2 μM and near complete inhibition (~15% residual activity) is observed with >50 μM HQNO. At 50 μM MD, HQNO inhibition is observed with an IC50 value of 54.3±1.2 μM[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 445.8±37.0 °C at 760 mmHg |
| Melting Point | 156-157ºC |
| Molecular Formula | C16H21NO2 |
| Molecular Weight | 259.343 |
| Flash Point | 223.4±26.5 °C |
| Exact Mass | 259.157227 |
| PSA | 42.23000 |
| LogP | 3.18 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.558 |
| Hazard Codes | Xi |
|---|---|
| RTECS | VC5890000 |
| HS Code | 2933499090 |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |