5688-82-4
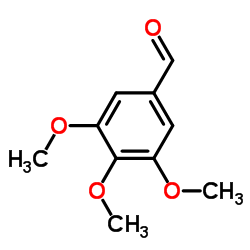
| Name | 2-[(3,4,5-trimethoxyphenyl)methylidene]propanedinitrile |
|---|---|
| Synonyms |
EINECS 227-155-9
((3,4,5-Trimethoxyphenyl)methylene)malononitrile (3,4,5-trimethoxybenzylidene)malononitrile MALONONITRILE,(3,4,5-TRIMETHOXYBENZYLIDENE) 2-(3,4,5-trimethoxybenzylidene)malononitrile (3,4,5-trimethoxybenzylidene)propanedinitrile ((3,4,5-TRIMETHOXYPHENYL)METHYLENE)METHANE-1,1-DICARBONITRILE |
| Density | 1.188g/cm3 |
|---|---|
| Boiling Point | 406ºC at 760 mmHg |
| Molecular Formula | C13H12N2O3 |
| Molecular Weight | 244.24600 |
| Flash Point | 164.7ºC |
| Exact Mass | 244.08500 |
| PSA | 75.27000 |
| LogP | 2.14296 |
| Index of Refraction | 1.56 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~10% 
5688-82-4 |
| Literature: Khan, Faiz Ahmed; Dash, Jyotirmayee; Satapathy, Rashmirekha; Upadhyay, Sarasij K. Tetrahedron Letters, 2004 , vol. 45, # 15 p. 3055 - 3058 |
|
~53% 
5688-82-4 |
| Literature: Sidhu, Anjali; Sharma; Rai, Mangat Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2010 , vol. 49, # 2 p. 247 - 250 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |