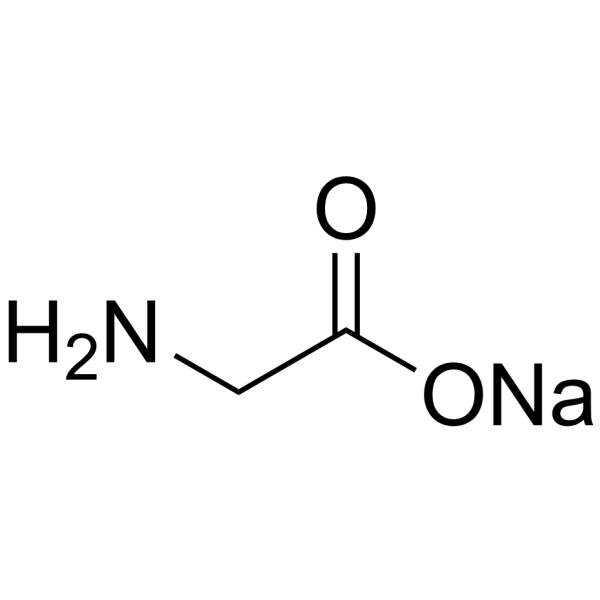
2441-41-0
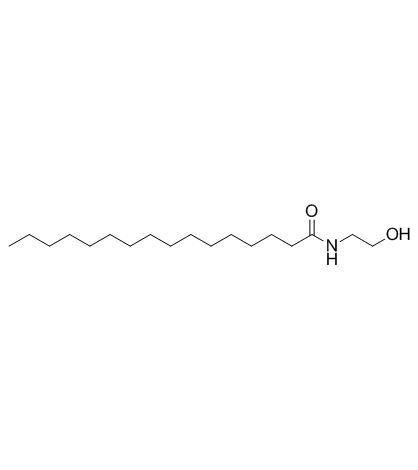
| Name | 2-(hexadecanoylamino)acetic acid |
|---|---|
| Synonyms |
N-Ethanoyl-Hexadecanamide
PALGLY N-hexadecanoyl glycine Palmitoylglycine QV1MV15 N-palmitatoylglycyne N-(1-Oxohexadecyl)glycine N-Hexadecanoylglycine Glycine, N-(1-oxohexadecyl)- Hexadecanoylaminoacetic acid N-palmitoyl glycine Hexadecanoylaminoacetic acid,N-palmitoyl glycine 2-(Hexadecanoylamino)acetic acid N-palmitoyl glycine N-Palmitoylglycine |
| Description | Palmitoylglycine, a novel endogenous lipid, acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Palmitoylglycine induces transient influx of calcium followed by nitric oxide production via calcium-sensitive nitric-oxide synthase enzymes. Palmitoylglycine potently inhibits heat-evoked firing of nociceptive neurons in rat dorsal horn[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 491.8±28.0 °C at 760 mmHg |
| Molecular Formula | C18H35NO3 |
| Molecular Weight | 313.48 |
| Flash Point | 251.2±24.0 °C |
| Exact Mass | 313.261688 |
| PSA | 66.40000 |
| LogP | 6.03 |
| Appearance | white to off-white |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.468 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >10mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2924199090 |
|
~76% 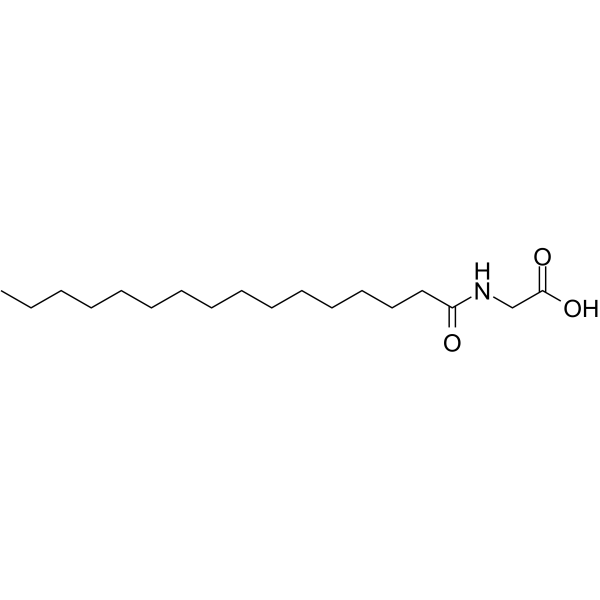
2441-41-0 |
| Literature: McIlhinney; Harvey Journal of Mass Spectrometry, 1995 , vol. 30, # 6 p. 900 - 910 |
|
~% 
2441-41-0 |
| Literature: Dang, Hung The; Kang, Gyeoung Jin; Yoo, Eun Sook; Hong, Jongki; Choi, Jae Sue; Kim, Hyung Sik; Chung, Hae Young; Jung, Jee H. Bioorganic and Medicinal Chemistry, 2011 , vol. 19, # 4 p. 1520 - 1527 |
|
~% 
2441-41-0 |
| Literature: Duarte, Rodrigo Da Costa; Ongaratto, Renata; Piovesan, Luciana Almeida; De Lima, Vania Rodrigues; Soldi, Valdir; Merlo, Aloir Antonio; D'Oca, Marcelo G. Montes Tetrahedron Letters, 2012 , vol. 53, # 19 p. 2454 - 2460 |
|
~% 
2441-41-0 |
| Literature: Bondi; Frankl Biochemische Zeitschrift, 1909 , vol. 17, p. 552 |
|
Detail
|
| Literature: Conopco, Inc., d/b/a UNILEVER Patent: US2007/299269 A1, 2007 ; Location in patent: Page/Page column 5-6; 7 ; |
|
~% 
2441-41-0 |
| Literature: Iyer, Venkataraman N.; Sheth, Geeta N.; Subrahmanyam, V. V. R. Journal of the Indian Chemical Society, 1982 , vol. 59, # 7 p. 856 - 859 |
|
~% 
2441-41-0 |
| Literature: KYUSHU UNIVERSITY; NISSAN CHEMICAL INDUSTRIES, LTD. Patent: US2012/35108 A1, 2012 ; |
|
~% 
2441-41-0 |
| Literature: Duarte, Rodrigo Da Costa; Ongaratto, Renata; Piovesan, Luciana Almeida; De Lima, Vania Rodrigues; Soldi, Valdir; Merlo, Aloir Antonio; D'Oca, Marcelo G. Montes Tetrahedron Letters, 2012 , vol. 53, # 19 p. 2454 - 2460 |
|
~% 
2441-41-0 |
| Literature: Abderhalden; Funk Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1910 , vol. 65, p. 66 |
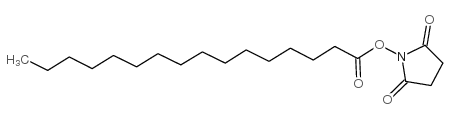
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |