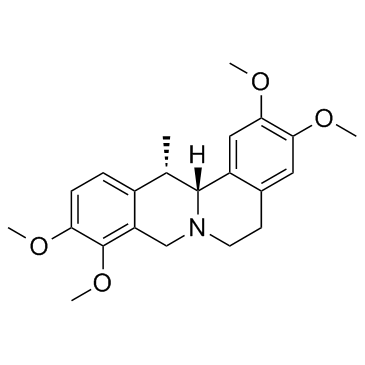
30045-16-0
| Name | Dehydrocorydaline |
|---|---|
| Synonyms |
dehydrocorydalmine
dehydeocorydaline 5,6-Dihydro-2,3,9,10-tetramethoxy-13-methyldibenzo[a,g]quinolizinium 2,3,9,10-Tetramethoxy-13-methyl-5,6-dihydro-isochino[3,2-a]isochinolinylium Dehydrocorydalin 13-Methyl-2,3,9,10-tetramethoxy-5,6-dihydrodibenzo[a,g]quinolizinium 13-methylpalmatine 2,3,9,10-tetramethoxy-13-methyl-5,6-dihydro-isoquino[3,2-a]isoquinolinylium |
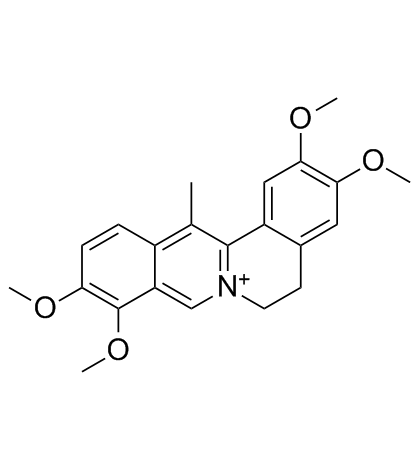
| Description | Dehydrocorydaline (13-Methylpalmatine) is an alkaloid isolated from traditional Chinese herb Corydalis yanhusuo W.T. Wang. Dehydrocorydaline regulates protein expression of Bax, Bcl-2; activates caspase-7, caspase-8, and inactivates PARP. |
|---|---|
| Related Catalog | |
| Target |
Bcl-2 Bax Caspase-7 Caspase-8 PARP |
| In Vitro | Dehydrocorydaline (0-200 μM) treatment significantly inhibits the growth of MCF-7 cells in a dose-dependent manner. The cell viability is decreased by approximate 40% after 24 h of 200 μM Dehydrocorydaline[1]. Dehydrocorydaline (0-200 μM)dose-dependently increases Bax protein expression and decreases Bcl-2 protein expression[1]. Dehydrocorydaline (0-200 μM)induces activation of caspase-7,-8 and the cleavage of PARP without affecting caspase-9[1]. |
| In Vivo | Dehydrocorydaline manifests a low acute toxicity with an LD50 of about 277.5±19.0 mg/kg body weight in mice following oral administration and 21.1±1.4 mg/kg for intraperitoneal injection[2]. |
| References |
| Melting Point | 170-173℃ |
|---|---|
| Molecular Formula | C22H24NO4+ |
| Molecular Weight | 366.43000 |
| Exact Mass | 366.17100 |
| PSA | 40.80000 |
| LogP | 3.69320 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
|
~% 
30045-16-0 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 253, p. 266,270 Archiv der Pharmazie (Weinheim, Germany), , vol. 256, p. 155,185 |
|
~% 
30045-16-0 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 256, p. 155,185 |
|
~% 
30045-16-0 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 243, p. 181 Journal of the Chemical Society, , vol. 71, p. 663 |
|
~% 
30045-16-0 |
| Literature: Journal of the Chemical Society, , vol. 71, p. 663 |
|
~% 
30045-16-0 |
| Literature: Archiv der Pharmazie (Weinheim, Germany), , vol. 234, p. 505 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |