2-Thiazolecarboxaldehyde
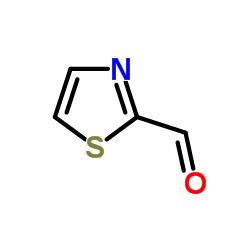
2-Thiazolecarboxaldehyde structure
|
Common Name | 2-Thiazolecarboxaldehyde | ||
|---|---|---|---|---|
| CAS Number | 10200-59-6 | Molecular Weight | 113.138 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 218.3±23.0 °C at 760 mmHg | |
| Molecular Formula | C4H3NOS | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 85.8±22.6 °C | |
| Name | 2-Thiazolecarboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 218.3±23.0 °C at 760 mmHg |
| Molecular Formula | C4H3NOS |
| Molecular Weight | 113.138 |
| Flash Point | 85.8±22.6 °C |
| Exact Mass | 112.993530 |
| PSA | 58.20000 |
| LogP | 0.50 |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.621 |
| Storage condition | Refrigerator (+4°C) |
| Water Solubility | soluble |
| Hazard Codes | Xn:Harmful; |
|---|---|
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S36/37/39-S26-S23 |
| WGK Germany | 3 |
| HS Code | 2934100090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Design, synthesis, antinociceptive and anti-inflammatory activities of novel piroxicam analogues.
Molecules 17(12) , 14126-45, (2012) In this paper we report the design, synthesis, antinociceptive and anti-inflammatory activities of a series of benzothiazine N-acylhydrazones 14a–h, planned by structural modification of piroxicam (1)... |
|
|
Asymmetric synthesis of an N-acylpyrrolidine for inhibition of HCV polymerase.
J. Org. Chem. 73(8) , 3094-102, (2008) A practical asymmetric synthesis of a highly substituted N-acylpyrrolidine on multi-kilogram scale is described. The key step in the construction of the three stereocenters is a [3+2] cycloaddition of... |
|
|
New highly active taxoids from 9 beta-dihydrobaccatin-9,10-acetals. Part 5.
Bioorg. Med. Chem. Lett. 14(12) , 3209-3215, (2004) To improve the metabolic stability of 3, which exhibited both in vitro antitumor activity and in vivo efficacy by both iv and po administration, we designed and synthesized new taxane analogues. Most ... |
| 2-Thiazolecarboxaldehyde |
| MFCD00142924 |
| Thiazole-2-carbaldehyde |
| 1,3-Thiazole-2-carbaldehyde |
| 2-Formylthiazole |
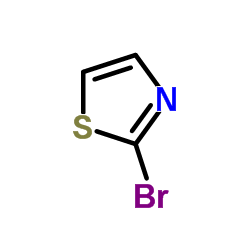 CAS#:3034-53-5
CAS#:3034-53-5 CAS#:68-12-2
CAS#:68-12-2 CAS#:288-47-1
CAS#:288-47-1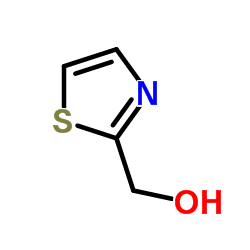 CAS#:14542-12-2
CAS#:14542-12-2 CAS#:134271-22-0
CAS#:134271-22-0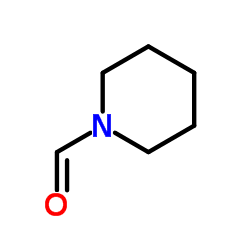 CAS#:2591-86-8
CAS#:2591-86-8 CAS#:40610-14-8
CAS#:40610-14-8 CAS#:4394-85-8
CAS#:4394-85-8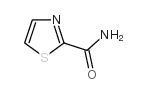 CAS#:16733-85-0
CAS#:16733-85-0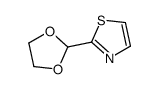 CAS#:24295-04-3
CAS#:24295-04-3 CAS#:1452-16-0
CAS#:1452-16-0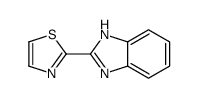 CAS#:3574-94-5
CAS#:3574-94-5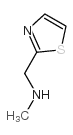 CAS#:144163-68-8
CAS#:144163-68-8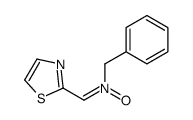 CAS#:162365-53-9
CAS#:162365-53-9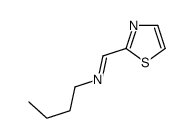 CAS#:882690-74-6
CAS#:882690-74-6 CAS#:845781-29-5
CAS#:845781-29-5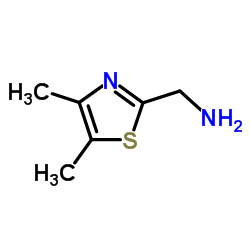 CAS#:89601-18-3
CAS#:89601-18-3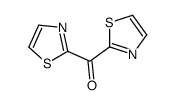 CAS#:55707-55-6
CAS#:55707-55-6
