Malonoben
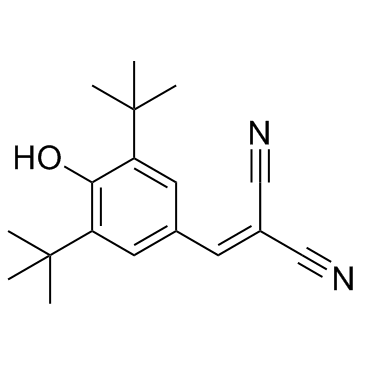
Malonoben structure
|
Common Name | Malonoben | ||
|---|---|---|---|---|
| CAS Number | 10537-47-0 | Molecular Weight | 282.380 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 386.8±37.0 °C at 760 mmHg | |
| Molecular Formula | C18H22N2O | Melting Point | 141-143℃ (ethanol ) | |
| MSDS | Chinese USA | Flash Point | 187.7±26.5 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of MalonobenTyrphostin A9(AG 17), a tyrosine kinase inhibitor, is a potent inducer of mitochondrial fission. Tyrphostin A9 emerged as the most potent and selective of 51 tyrosine kinase inhibitors tested against the TNF-induced respiratory burst. IC50 value: Target: Multi tyrosine kinaseTyrphostin A9 inhibited TNF-induced tyrosine phosphorylation of pyk2 without blocking the cells' bactericidal activity. Tyrphostin A9 is a PDGF receptor tyrosine kinase inhibitor (IC50 = 500 nM). Recent findings suggest that signaling via PDGF receptor tyrosine kinases is not necessary for the shift of the smooth muscle cells from a contractile to a synthetic phenotype. On the other hand these enzymes apparently carry out important functions in the control of intracellular membrane traffic and cell division. |
| Name | malonoben |
|---|---|
| Synonym | More Synonyms |
| Description | Tyrphostin A9(AG 17), a tyrosine kinase inhibitor, is a potent inducer of mitochondrial fission. Tyrphostin A9 emerged as the most potent and selective of 51 tyrosine kinase inhibitors tested against the TNF-induced respiratory burst. IC50 value: Target: Multi tyrosine kinaseTyrphostin A9 inhibited TNF-induced tyrosine phosphorylation of pyk2 without blocking the cells' bactericidal activity. Tyrphostin A9 is a PDGF receptor tyrosine kinase inhibitor (IC50 = 500 nM). Recent findings suggest that signaling via PDGF receptor tyrosine kinases is not necessary for the shift of the smooth muscle cells from a contractile to a synthetic phenotype. On the other hand these enzymes apparently carry out important functions in the control of intracellular membrane traffic and cell division. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 386.8±37.0 °C at 760 mmHg |
| Melting Point | 141-143℃ (ethanol ) |
| Molecular Formula | C18H22N2O |
| Molecular Weight | 282.380 |
| Flash Point | 187.7±26.5 °C |
| Exact Mass | 282.173218 |
| PSA | 67.81000 |
| LogP | 4.81 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.555 |
| Storage condition | Store at RT |
| Water Solubility | ethanol: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H331 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R23/24/25 |
| Safety Phrases | S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | OO3737000 |
| Hazard Class | 6.1 |
| HS Code | 2926909090 |
|
~84% 
Malonoben CAS#:10537-47-0 |
| Literature: Hori, Hitoshi; Nagasawa, Hideko; Ishibashi, Masaki; Uto, Yoshihiro; Hirata, Akihiko; Saijo, Kouichi; Ohkura, Kazuto; Kirk, Kenneth L; Uehara, Yoshimasa Bioorganic and Medicinal Chemistry, 2002 , vol. 10, # 10 p. 3257 - 3265 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Pyk2 uncouples metabotropic glutamate receptor G protein signaling but facilitates ERK1/2 activation.
Mol. Brain 3 , 4, (2010) Group I metabotropic glutamate receptors (mGluRs) are coupled via Galphaq/11 to the activation of phospholipase Cbeta, which hydrolyzes membrane phospholipids to form inositol 1,4,5 trisphosphate and ... |
|
|
Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage.
FASEB J. 30 , 286-300, (2016) Itaconate is a nonamino organic acid exhibiting antimicrobial effects. It has been recently identified in cells of macrophage lineage as a product of an enzyme encoded by immunoresponsive gene 1 (Irg1... |
|
|
Vesicular transport and apotransferrin in intestinal iron absorption, as shown in the Caco-2 cell model.
Am. J. Physiol. Gastrointest. Liver Physiol. 290 , G301-G309, (2006) The potential roles of vesicular transport and apotransferrin (entering from the blood) in intestinal Fe absorption were investigated using Caco-2 cell monolayers with tight junctions in bicameral cha... |
| (3,5-Di-tert-butyl-4-hydroxybenzyliden)malononitril |
| 2-(3,5-di-tert-butyl-4-hydroxybenzylidene)malononitrile |
| Malonoben |
| MFCD00209853 |
| 2-[(3,5-ditert-butyl-4-hydroxyphenyl)methylidene]propanedinitrile |
| (3,5-Di-tert-butyl-4-hydroxybenzylidene)malononitrile |
| Tyrphostin AG17 |
| Tyrphostin 9 |
| 2-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]methylene]propanedinitrile |
| [4-Hydroxy-3,5-bis(2-methyl-2-propanyl)benzylidene]malononitrile |
| GCP 5126 |
| Propanedinitrile, 2-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]methylene]- |
| (3,5-di-tert-butyl-4-hydroxybenzylidene)propanedinitrile |
| Caswell No. 291AA |
| [(3,5-di-tert-butyl-4-hydroxyphenyl)methylidene]propanedinitrile |
| 3,5-di-tert-butyl-4-hydroxybenzylidene-malononitrile |
| tyrphostin A9 |