Trimethylgermanium bromide
Modify Date: 2024-01-02 13:02:38
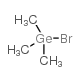
Trimethylgermanium bromide structure
|
Common Name | Trimethylgermanium bromide | ||
|---|---|---|---|---|
| CAS Number | 1066-37-1 | Molecular Weight | 197.64800 | |
| Density | 1.54 g/mL at 25 °C(lit.) | Boiling Point | 114 °C(lit.) | |
| Molecular Formula | C3H9BrGe | Melting Point | −25 °C(lit.) | |
| MSDS | N/A | Flash Point | 99 °F | |
| Name | bromo(trimethyl)germane |
|---|---|
| Synonym | More Synonyms |
| Density | 1.54 g/mL at 25 °C(lit.) |
|---|---|
| Boiling Point | 114 °C(lit.) |
| Melting Point | −25 °C(lit.) |
| Molecular Formula | C3H9BrGe |
| Molecular Weight | 197.64800 |
| Flash Point | 99 °F |
| Exact Mass | 197.91000 |
| LogP | 2.21620 |
| Vapour Pressure | 24mmHg at 25°C |
| Index of Refraction | n20/D 1.47(lit.) |
| Storage condition | Flammables area |
Synonym:None Known Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 10 34 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Flammable. Causes burns.Light sensitive.Moisture sensitive.Corrosive. Potential Health Effects Eye: Causes eye burns. May cause chemical conjunctivitis and corneal damage. Skin: Causes skin burns. May cause cyanosis of the extremities. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color. Ingestion: May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. Ingestion of large amounts may cause CNS depression. May cause systemic effects. Inhalation: Causes chemical burns to the respiratory tract. Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. May cause systemic effects. May cause burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting. Chronic: Effects may be delayed. Section 4 - FIRST AID MEASURES Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes). Skin: Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes. Ingestion: Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Inhalation: Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air. Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Water hydrolyzes material liberating acidic gas which in contact with metal surfaces can generate flammable and/or explosive hydrogen gas. Runoff from fire control or dilution water may cause pollution. Extinguishing Media: Use water spray to cool fire-exposed containers. Water may be ineffective. DO NOT USE WATER! In case of fire, use carbon dioxide, dry chemical powder or appropriate foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Isolate area and deny entry. Provide ventilation. Do not get water inside containers. A vapor suppressing foam may be used to reduce vapors. Section 7 - HANDLING and STORAGE Handling: Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Do not ingest or inhale. Store protected from light. Do not allow contact with water. Use only in a chemical fume hood. Discard contaminated shoes. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Keep from contact with moist air and steam. Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a cool, dry place. Keep container closed when not in use. Store in a tightly closed container. Flammables-area. Store protected from moisture. Store protected from light. Store under nitrogen. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels. Exposure Limits CAS# 1066-37-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Color: colorless Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: 114 deg C @ 760 mmHg Freezing/Melting Point: -25 deg C Autoignition Temperature: Not available. Flash Point: 37 deg C ( 98.60 deg F) Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: Specific Gravity/Density: 1.540 Molecular Formula: C3H9BrGe Molecular Weight: 197.60 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Moisture sensitive. Light sensitive. Conditions to Avoid: Incompatible materials, light, ignition sources, excess heat, exposure to moist air or water. Incompatibilities with Other Materials: Oxidizing agents, strong bases. Hazardous Decomposition Products: Carbon monoxide, carbon dioxide, hydrogen bromide, oxides of germanium. Hazardous Polymerization: Has not been reported Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 1066-37-1 unlisted. LD50/LC50: Not available. Carcinogenicity: Trimethylgermanium Bromide - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Ecotoxicity: Fish: Pseudomonas putida: Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: Corrosive liquid, flammable, n.o.s.* Hazard Class: 8 UN Number: 2920 Packing Group: II IMO Shipping Name: Corrosive liquid, flammable, n.o.s. Hazard Class: 8 UN Number: 2920 Packing Group: II RID/ADR Shipping Name: Corrosive Liquid, Flammable, N.O.S. Hazard Class: 8 UN Number: 2920 Packing group: Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: C Risk Phrases: R 10 Flammable. R 34 Causes burns. Safety Phrases: S 16 Keep away from sources of ignition - No smoking. S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 36/37/39 Wear suitable protective clothing, gloves and eye/face protection. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 1066-37-1: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 1066-37-1 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 1066-37-1 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | C:Corrosive; |
|---|---|
| Risk Phrases | R10;R34 |
| Safety Phrases | S16-S26-S27-S36/37/39-S45 |
| RIDADR | UN 2920 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 3 |
| HS Code | 2931900090 |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
| Trimethylgermanium bromide |
| EINECS 213-913-6 |
| Bromotrimethylgermane |
| Trimethylgermanium-bromid |
| MFCD00000037 |
| Brom-trimethyl-germanium |
| trimethylbromogermane |