(S)-(+)-2-(Dibenzylamino)-3-phenyl-1-propanol

(S)-(+)-2-(Dibenzylamino)-3-phenyl-1-propanol structure
|
Common Name | (S)-(+)-2-(Dibenzylamino)-3-phenyl-1-propanol | ||
|---|---|---|---|---|
| CAS Number | 111060-52-7 | Molecular Weight | 331.45100 | |
| Density | 1.111g/cm3 | Boiling Point | 488ºC at 760mmHg | |
| Molecular Formula | C23H25NO | Melting Point | 72-74 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 191.3ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | (s)-(+)-2-dibenzylamino-3-phenyl-1-propanol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.111g/cm3 |
|---|---|
| Boiling Point | 488ºC at 760mmHg |
| Melting Point | 72-74 °C(lit.) |
| Molecular Formula | C23H25NO |
| Molecular Weight | 331.45100 |
| Flash Point | 191.3ºC |
| Exact Mass | 331.19400 |
| PSA | 23.47000 |
| LogP | 4.29240 |
| Vapour Pressure | 2.45E-10mmHg at 25°C |
| Index of Refraction | 1.613 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922199090 |
| Precursor 8 | |
|---|---|
| DownStream 5 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Preparation of Aminoalkyl Chlorohydrin Hydrochlorides: Key Building Blocks for Hydroxyethylamine-Based HIV Protease Inhibitors.
J. Org. Chem. 61 , 3635, (1996) Enantiomerically pure N,N-dibenzyl-alpha-amino aldehydes reacted with (chloromethyl)lithium, generated in situ from bromochloromethane and lithium metal, to give predominantly erythro aminoalkyl epoxi... |
|
|
Stereoselective Synthesis of HIV-1 Protease Inhibitor, DMP 323.
J. Org. Chem. 61 , 444, (1996) DMP 323, a potent HIV-1 protease inhibitor, has been synthesized by an efficient stereoselective process, amenable to large scale preparations. The core C(2) symmetric diol was synthesized by a stereo... |
|
|
Cyclic HIV protease inhibitors: synthesis, conformational analysis, P2/P2' structure-activity relationship, and molecular recognition of cyclic ureas.
J. Med. Chem. 39 , 3514, (1996) High-resolution X-ray structures of the complexes of HIV-1 protease (HIV-1PR) with peptidomimetic inhibitors reveal the presence of a structural water molecule which is hydrogen bonded to both the mob... |
| (2S)-2-(dibenzylamino)-3-phenylpropan-1-ol |
| MFCD00191984 |
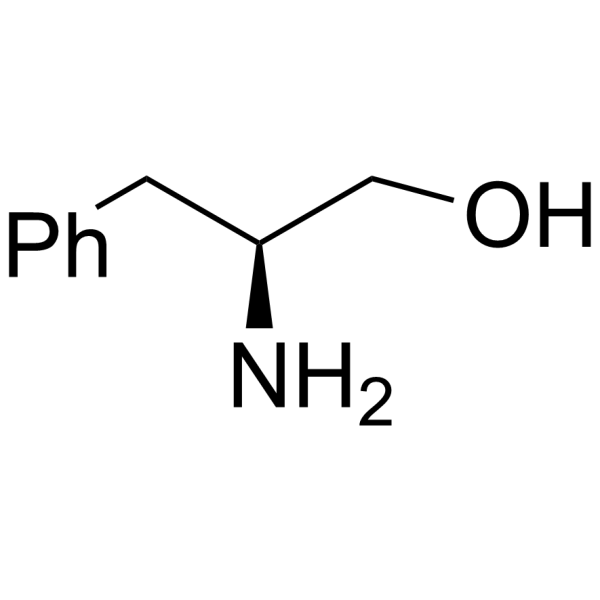 CAS#:3182-95-4
CAS#:3182-95-4 CAS#:100-39-0
CAS#:100-39-0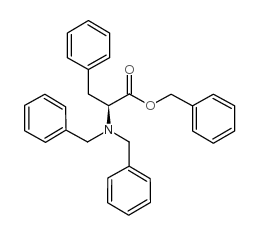 CAS#:111138-83-1
CAS#:111138-83-1 CAS#:100-52-7
CAS#:100-52-7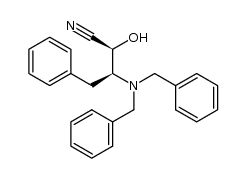 CAS#:118970-37-9
CAS#:118970-37-9 CAS#:63-91-2
CAS#:63-91-2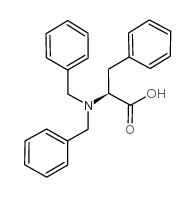 CAS#:95437-43-7
CAS#:95437-43-7![2S-[bis(phenylmethyl)amino]benzenepropanaldehyde Structure](https://image.chemsrc.com/caspic/122/123054-12-6.png) CAS#:123054-12-6
CAS#:123054-12-6 CAS#:98760-08-8
CAS#:98760-08-8 CAS#:165727-45-7
CAS#:165727-45-7![tert-Butyl [(1S,2R)-1-Benzyl-2-hydroxy-3-(isobutylamino)propyl]carbamate structure](https://image.chemsrc.com/caspic/176/160232-08-6.png) CAS#:160232-08-6
CAS#:160232-08-6 CAS#:17585-69-2
CAS#:17585-69-2 CAS#:2566-22-5
CAS#:2566-22-5