Indole-5-carboxaldehyde
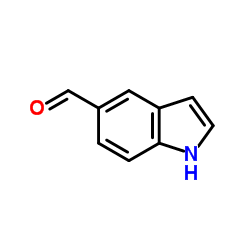
Indole-5-carboxaldehyde structure
|
Common Name | Indole-5-carboxaldehyde | ||
|---|---|---|---|---|
| CAS Number | 1196-69-6 | Molecular Weight | 145.158 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 339.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H7NO | Melting Point | 100-103 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 166.8±27.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Indole-5-carboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 339.1±15.0 °C at 760 mmHg |
| Melting Point | 100-103 °C(lit.) |
| Molecular Formula | C9H7NO |
| Molecular Weight | 145.158 |
| Flash Point | 166.8±27.8 °C |
| Exact Mass | 145.052765 |
| PSA | 32.86000 |
| LogP | 1.56 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.729 |
| Storage condition | Keep Cold |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and antifungal activity of novel streptochlorin analogues.
Eur. J. Med. Chem. 92 , 776-83, (2015) Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Bas... |
|
|
Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization.
Bioorg. Med. Chem. 19 , 4782-95, (2011) We previously reported the discovery of 2-aryl-4-benzoyl-imidazoles (ABI-I) as potent antiproliferative agents for melanoma. To further understand the structural requirements for the potency of ABI an... |
|
|
Synthesis and biological evaluation of achiral indole-substituted titanocene dichloride derivatives.
Int J Med Chem 2012 , 905981, (2015) Six new titanocene compounds have been isolated and characterised. These compounds were synthesised from their fulvene precursors using Super Hydride (LiBEt3H) followed by transmetallation with titani... |
| MFCD02093664 |
| 5-Formylindole |
| 1H-Indole-5-carboxaldehyde |
| Indole-5-carboxaldehyde |
| 1H-Indole-5-carbaldehyde |
 CAS#:16066-91-4
CAS#:16066-91-4 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:1075-25-8
CAS#:1075-25-8 CAS#:1670-81-1
CAS#:1670-81-1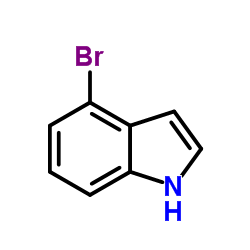 CAS#:10075-50-0
CAS#:10075-50-0 CAS#:68-12-2
CAS#:68-12-2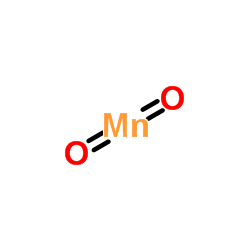 CAS#:1313-13-9
CAS#:1313-13-9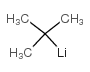 CAS#:594-19-4
CAS#:594-19-4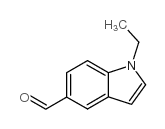 CAS#:944893-74-7
CAS#:944893-74-7 CAS#:90923-75-4
CAS#:90923-75-4 CAS#:21005-48-1
CAS#:21005-48-1 CAS#:63263-88-7
CAS#:63263-88-7 CAS#:166670-56-0
CAS#:166670-56-0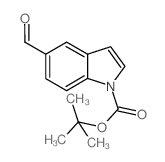 CAS#:279256-09-6
CAS#:279256-09-6 CAS#:15861-24-2
CAS#:15861-24-2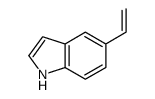 CAS#:77132-99-1
CAS#:77132-99-1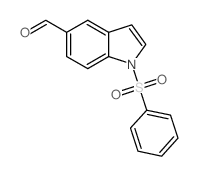 CAS#:671215-62-6
CAS#:671215-62-6
