Gedatolisib (PF-05212384, PKI-587)
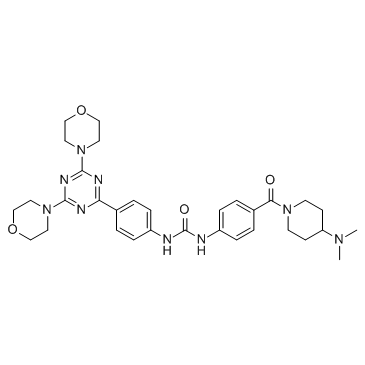
Gedatolisib (PF-05212384, PKI-587) structure
|
Common Name | Gedatolisib (PF-05212384, PKI-587) | ||
|---|---|---|---|---|
| CAS Number | 1197160-78-3 | Molecular Weight | 615.726 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C32H41N9O4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of Gedatolisib (PF-05212384, PKI-587)Gedatolisib (PKI-587) is a highly potent dual inhibitor of PI3Kα, PI3Kγ, and mTOR with IC50s of 0.4 nM, 5.4 nM and 1.6 nM, respectively. PKI-587 is equally effective in both complexes of mTOR, mTORC1 and mTORC2. |
| Name | 1-[4-[4-(dimethylamino)piperidine-1-carbonyl]phenyl]-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea |
|---|---|
| Synonym | More Synonyms |
| Description | Gedatolisib (PKI-587) is a highly potent dual inhibitor of PI3Kα, PI3Kγ, and mTOR with IC50s of 0.4 nM, 5.4 nM and 1.6 nM, respectively. PKI-587 is equally effective in both complexes of mTOR, mTORC1 and mTORC2. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:0.4 nM (IC50) PI3Kβ:6 nM (IC50) PI3Kδ:6 nM (IC50) PI3Kγ:5.4 nM (IC50) PI3Kα-H1047R:0.6 nM (IC50) PI3Kα-E545K:0.6 nM (IC50) mTOR:1.6 nM (IC50) mTORC1 mTORC2 |
| In Vitro | Gedatolisib (PKI-587) shows good potency in cell growth inhibition assays using MDA-361 and PC3-MM2 cell lines. Tumor cell growth inhibition by Gedatolisib (PKI-587) correlated with suppression of phosphorylation of PI3K/mTOR signaling pathway proteins in MDA-361 tumor cells. Gedatolisib (PKI-587) prevents the phosphorylation of Akt (pAkt) at threonine 308 (T308; IC50=8 nM) (phosphoblot IC50 values are determined by densitometric scans of Western blots) and induces cleaved PARP at 30 nM. Cleaved PARP is a marker for cells undergoing apoptosis. Full activation of Akt kinase occurs when the mTOR containing mammalian target of rapamycin 2 (TORC2) protein complex phosphorylates Akt at serine 473 (S473). Phophorylation of Akt kinase effector proteins GSK3 kinase, endothelial nitric oxide synthase (ENOS), and prolinerich Akt substrate (PRAS 40) is also suppressed by Gedatolisib (PKI-587) at concentrations less than 30 nM. The inhibition of mTOR containing TORC1 kinase activity by Gedatolisib (PKI-587) is demonstrated by suppression of p70S6K and 4EBP1 phosphorylation at concentrations less than 30 nM by Gedatolisib (PKI-587)[1]. Gedatolisib (PKI-587) effectively inhibits proliferation of NPC cells by blocking the PI3K/mTOR/S6/4EBP1 signaling pathway. Because administration of 0.03 μM Gedatolisib (PKI-587) can significantly inhibit the signaling pathway with little effect on cell viability after 24 hours, those concentrations of PKI-587 are chosen for additional experiments. Treatment with GSK2126458 or Gedatolisib (PKI-587) alone leads to an increased percentage of cells in G1phase, whereas IR alone leads to a G2-M arrest. Strikingly, the combination of IR with GSK2126458 (0.003 μM) or PKI-587Gedatolisib (PKI-587) (0.03 μM) induces a further arrest in the G2-M phase, which highlights the potential radiosensitizing capability of these reagents. Further investigation indicated that the level of cyclin D1 is decreased 24 hours after treatment with GSK2126458 or Gedatolisib (PKI-587). Meanwhile the expression of p21 and p27 are increased 24 hours post-IR. The combination of IR with GSK2126458 or Gedatolisib (PKI-587) further increases the level of p21 and p27, accompanied with dramatic decrease of cyclin D1[2]. |
| In Vivo | When Gedatolisib (PKI-587) is administered to nude mice at 25 mg/kg iv (in 5% dextrose [D5/W], 0.3% lactic acid, pH 3.5), low plasma clearance (7 (mL/min)/kg) compared to liver blood flow (90 (mL/min)/kg), high volume of distribution (7.2 L/kg) compared to plasma volume (0.7 L/kg), and long half-life, (14.4 h) are observed. Low clearance indicated minimal metabolism, and high volume of distribution is suggestive of extensive tissue distribution in nude mice. When Gedatolisib (PKI-587) is administered iv (single dose) at 25 mg/kg, it suppresses pAkt (T308 and S473) for up to 36 h and induces cleaved PARP up to 18 h in MDA-361 tumors staged in nude mice. Gedatolisib (PKI-587) administered iv at 20 mg/kg on an intermittent regimen (days 1, 5, 9) caused regression of large staged (~900 mm3) tumors. This effect is more pronounced than that observed for paclitaxel (Taxol) given at 60 mg/kg (single dose, ip). In subsequent in vivo studies the minimum efficacious dose (MED) of PKI-587 is determined to be 3 mg/kg against MDA-361 tumors and maximum tolerated single dose (MTD) is determined to be 30 mg/kg[1]. To assess the antitumor effects of dual PI3K/mTOR inhibitors in combination with radiation therapy in vivo, the 5-8F xenografted model is used, which is a well-established model of NPC using one of the NPC cell lines from our in vitro experiments. GSK2126458 or Gedatolisib (PKI-587) alone has a modest antitumor activity and that the ionizing radiation (IR) shows better inhibition of tumor growth. The combination of IR with GSK2126458 or Gedatolisib (PKI-587) results in >50% reduction in xenograft volume and tumor regrowth delay when compared with IR alone[2]. |
| Cell Assay | Cell proliferation is measured using the MTT dye reduction method. In brief, tumor cells (2×103/100 μL/well) are plated into each well of 96-well plates in RMPI-1640 with 10% FBS and incubated for 24 hours. Culture medium containing several concentrations of GSK2126458 (0-3 μM) or Gedatolisib (PKI-587) (0-3 μM) are added to each well, and the cells are incubated for 24 to 72 hours. Next, 50 μL of MTT (2 mg/mL) is added to each well, and cells are incubated for 2 hours at 37°C. The medium containing MTT solution is removed, and the dark blue crystals are dissolved by adding 100 μL DMSO. The absorbance is measured with a microplate reader at test and reference wavelengths of 550 and 630 nm, respectively. The percentage growth is determined relative to untreated controls. Each experiment is performed at least three times, each with triplicate samples[2]. |
| Animal Admin | Mice[2] Suspensions of 5×106/0.2 mL 5-8F cells are injected subcutaneously into the right hindlimbs of 5- to 7-week-old female BALB/c-nu/nu nude mice. When tumor volumes reach 200 mm3, mice are randomly assigned to control and treated groups (5 mice per group). The treated groups receive 300 μg/kg GSK2126458, 25 mg/kg PKI-587, IR, 300 μg/kg GSK2126458 combined with IR, or 25 mg/kg Gedatolisib (PKI-587) combined with IR. GSK2126458 is administered by intragastric administration once daily for 5 consecutive days each week and Gedatolisib (PKI-587) is administered by intravenous injection via tail vein once per 5 days. Mice in the IR groups are irradiated with 2 Gy every other day for four treatments. In combination therapy, GSK2126458 or Gedatolisib (PKI-587) is administered 2 hours before IR exposure. Tumor sizes are calculated every 2 days. Tumor regrowth delay is expressed as the time in days for xenografts treated with GSK2126458, Gedatolisib (PKI-587), or radiation to grow from 200 to 1,000 mm3 in volume minus the time in days for untreated tumors to reach the same size. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C32H41N9O4 |
| Molecular Weight | 615.726 |
| Exact Mass | 615.328125 |
| PSA | 128.29000 |
| LogP | -0.76 |
| Index of Refraction | 1.670 |
| Storage condition | 2~8°C |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 29349990 |
|
~90% 
Gedatolisib (PF... CAS#:1197160-78-3 |
| Literature: WYETH LLC; KHAFIZOVA, Gulnaz; POTOSKI, John, Richard Patent: WO2010/96619 A1, 2010 ; Location in patent: Page/Page column 29 ; WO 2010/096619 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| 1-(4-{[4-(Dimethylamino)-1-piperidinyl]carbonyl}phenyl)-3-{4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl}urea |
| PF-05212384 (PKI-587) |
| 1-(4-{[4-(dimethylamino)piperidin-1-yl]carbonyl}phenyl)-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)phenyl]urea |
| PKI 587 |
| cc-54 |
| Gedatolisib |
| N-[4-[[4-(Dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl]urea |
| PF 05212384 |
| PKI-587 |
| S2628_Selleck |
| 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea |
| 1-(4-{[4-(dimethylamino)piperidin-1-yl]carbonyl}phenyl)-3-[4-(4,6-dimorpholin-4-yl-1,3,5-triazine-2-yl)phenyl]urea |
| Urea, N-[4-[[4-(dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl]- |

![Benzoic acid,4-[[[[4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl]amino]carbonyl]amino]- structure](https://image.chemsrc.com/caspic/180/1197160-66-9.png)