magnesium dihydroxide
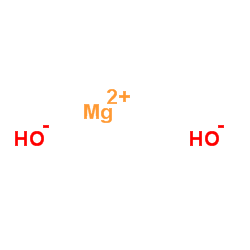
magnesium dihydroxide structure
|
Common Name | magnesium dihydroxide | ||
|---|---|---|---|---|
| CAS Number | 1309-42-8 | Molecular Weight | 58.320 | |
| Density | 2.36g/cm3 | Boiling Point | 100ºC at 760mmHg | |
| Molecular Formula | H2MgO2 | Melting Point | 350ºC ( Decomposes) | |
| MSDS | Chinese USA | Flash Point | will not burn | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | magnesium dihydroxide |
|---|---|
| Synonym | More Synonyms |
| Density | 2.36g/cm3 |
|---|---|
| Boiling Point | 100ºC at 760mmHg |
| Melting Point | 350ºC ( Decomposes) |
| Molecular Formula | H2MgO2 |
| Molecular Weight | 58.320 |
| Flash Point | will not burn |
| Exact Mass | 57.990520 |
| PSA | 40.46000 |
| Stability | Stable. |
| Water Solubility | 5 M HCl: 0.1 M, clear, yellow | 0.9 mg/100 mL (18 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36-37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | - |
| RTECS | OM3570000 |
|
~% 
magnesium dihyd... CAS#:1309-42-8 |
| Literature: Journal of Physical Chemistry, , vol. 97, # 24 p. 6449 - 6456 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Hydrotalcite composites for an effective fluoride buccal administration: a new technological approach.
Int. J. Pharm. 454(1) , 259-68, (2013) The aim of this work was to develop new mucoadhesive buccal patches containing an inorganic fluorinated compound, MgAl-F, intended for decay prevention. Firstly MgAl-F was synthesized and characterize... |
|
|
Evaluation of enantiomeric purity of magnesium-L-aspartate dihydrate.
J. Pharm. Biomed. Anal. 102 , 100-9, (2014) Magnesium supplementation in form of organic magnesium salts is a very popular practice. We examined the enantiomeric purity of "Magnesium aspartate dihydrate" monographed in the European Pharmacopeia... |
|
|
A kinetic study of struvite precipitation recycling technology with NaOH/Mg(OH)2 addition.
Bioresour. Technol. 143 , 519-24, (2013) Struvite precipitation recycling technology is received wide attention in removal ammonium and phosphate out of wastewater. While past study focused on process efficiency, and less on kinetics. The ki... |
| EINECS 215-170-3 |
| MFCD00011104 |
| magnesium,dihydroxide |
| Magnesium Hydroxide |