Zanamivir
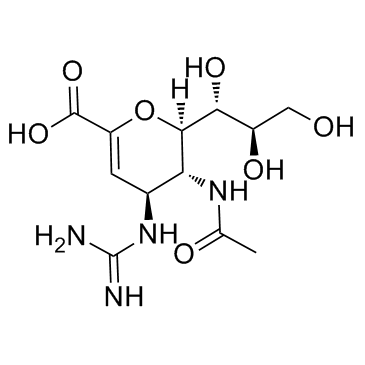
Zanamivir structure
|
Common Name | Zanamivir | ||
|---|---|---|---|---|
| CAS Number | 139110-80-8 | Molecular Weight | 332.310 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H20N4O7 | Melting Point | 256ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of ZanamivirZanamivir is an influenza viral neuraminidase inhibitor with IC50 values of 0.95 nM and 2.7 nM for influenza A and B, respectively. |
| Name | zanamivir |
|---|---|
| Synonym | More Synonyms |
| Description | Zanamivir is an influenza viral neuraminidase inhibitor with IC50 values of 0.95 nM and 2.7 nM for influenza A and B, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.95 nM (Influenza A); 2.7 nM (Influenza B)[1] |
| In Vitro | Zanamivir interacts with a group of amino acids in the active site of neuraminidase, which are conserved in all influenza A and B strains. Zanamivir blocks the action of neuraminidase, which prevents the cleavage of sialic acid on the cell receptors, thus preventing release and spread of the newly formed virions[2]. |
| In Vivo | Zanamivir has a poor bioavailability in oral administration, with only 4–17% of the agent. Oral delivery of zanamivir has been a problem due to its strong hydrophilic nature that limits its transport across the intestinal epithelium. Permeation enhancers such as sodium cholate, hydroxypropyl β-cyclodextrin could be used with zanamivir to enhance the intestinal permeability[3]. |
| Animal Admin | Rats: Formulations PO-SC (Zanamivir with SC for p.o.) and PO-C (Zanamivir control solution for p.o.) are administered orally at a Zanamivir dose of 10 mg/kg, and IV-R (reference Zanamivir saline solution for i.v.) is administered i.v. at a dose of 1 mg/kg to rats under conscious condition. Blood samples are collected prior to and at 0.5, 1, 2, 3, 4, 6, 8, and 24 hr after administration. At each sampling point, three rats from each group are sacrificed after blood collection to extract the lungs. The lungs are cleansed with saline after extraction of lungs from the rats through a chest incision. The lungs are then transferred into E-tube and stored in the freezer (-80°C) until analysis. Plasma samples are harvested by centrifugation at 1,500 × g for 10 min and stored at -20°C until analysis. The analysis of Zanamivir in both plasma and lungs is performed using before-mentioned LC-MS/MS method[3]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Melting Point | 256ºC (dec.) |
| Molecular Formula | C12H20N4O7 |
| Molecular Weight | 332.310 |
| Exact Mass | 332.133209 |
| PSA | 198.22000 |
| LogP | -4.13 |
| Index of Refraction | 1.679 |
| Storage condition | 2~8℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xn |
| Risk Phrases | 22-36/37/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| RTECS | RA9451000 |
|
Cochrane researchers continue to face challenges over access to data on flu drugs.
BMJ 346 , f3190, (2013)
|
|
|
Structure-based design and synthesis of C-1- and C-4-modified analogs of zanamivir as neuraminidase inhibitors.
J. Med. Chem. 56(3) , 671-84, (2013) In order to exploit the 430-cavity in the active sites of neuraminidases, 22 zanamivir analogs with C-1 and C-4 modification were synthesized, and their inhibitory activities against both group-1 (H5N... |
|
|
Mechanism-based covalent neuraminidase inhibitors with broad-spectrum influenza antiviral activity.
Science 340(6128) , 71-5, (2013) Influenza antiviral agents play important roles in modulating disease severity and in controlling pandemics while vaccines are prepared, but the development of resistance to agents like the commonly u... |
| 5-(Acetylamino)-4-[(aminoiminomethyl)amino]-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic Acid |
| 5-(acetylamino)-2,6-anhydro-4-carbamimidamido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid |
| 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid 4-Guanidino-Neu5Ac2en |
| (6R)-5-Acetamido-2,6-anhydro-4-carbamimidamido-3,4,5-trideoxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]-L-threo-hex-2-enonic acid |
| 5-(acetylamino)-4-{[amino(imino)methyl]amino}-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid |
| GANA |
| D-glycero-D-galacto-Non-2-enonic acid, 5-(acetylamino)-4-[(aminoiminomethyl)amino]-2,6-anhydro-3,4,5-trideoxy- |
| GG 167 |
| Zanamir |
| Zanamivir |
| Relenza |
| Zanamavir |
| Zanamivir Hydrate |
| 5-Acetamido-4-guanidino-2,3,4,5-tetradeoxy-D-glycero-D-galacto-non-2-enopyranosonic Acid |
| 5-Acetamido-2,6-anhydro-4-carbamimidamido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid |
| Unii-L6o3xi777i |
 CAS#:4023-02-3
CAS#:4023-02-3 CAS#:139110-70-6
CAS#:139110-70-6 CAS#:130525-58-5
CAS#:130525-58-5 CAS#:1184-90-3
CAS#:1184-90-3 CAS#:130525-62-1
CAS#:130525-62-1![methyl (3aR,4R,7aR)-2-methyl-4-(1S,2R,3-triacetoxypropyl)-3a,7a-dihydro-4H-pyrano[3,4-d]oxazole-6-carboxylate Structure](https://www.chemsrc.com/caspic/114/78850-37-0.png) CAS#:78850-37-0
CAS#:78850-37-0 CAS#:66356-40-9
CAS#:66356-40-9
