Trapidil
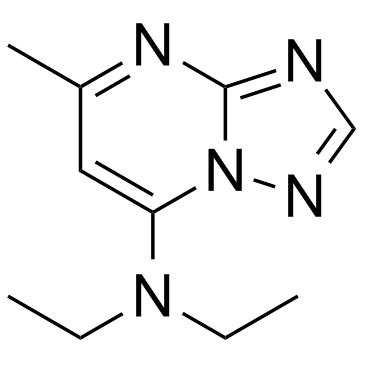
Trapidil structure
|
Common Name | Trapidil | ||
|---|---|---|---|---|
| CAS Number | 15421-84-8 | Molecular Weight | 205.260 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C10H15N5 | Melting Point | 98-99.4° (Pfeifer); 102-104° from heptane (Tenor) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of TrapidilTrapidil is a vasodilator, is an antiplatelet drug with specific platelet-derived growth factor. |
| Name | N,N-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine |
|---|---|
| Synonym | More Synonyms |
| Description | Trapidil is a vasodilator, is an antiplatelet drug with specific platelet-derived growth factor. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 98-99.4° (Pfeifer); 102-104° from heptane (Tenor) |
| Molecular Formula | C10H15N5 |
| Molecular Weight | 205.260 |
| Exact Mass | 205.132751 |
| PSA | 46.32000 |
| LogP | 1.45 |
| Index of Refraction | 1.626 |
| Storage condition | room temp |
| Water Solubility | H2O: ≥15mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~% 
Trapidil CAS#:15421-84-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 6, p. 583,585 |
|
~% 
Trapidil CAS#:15421-84-8 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 6, p. 583,585 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Protective effects of trapidil in ischemia-reperfusion injury due to testicular torsion and detorsion: an experimental study.
Int. J. Urol. 13(5) , 601-5, (2006) We aimed to detect the preventive effects of trapidil in ischemia-reperfusion (IR) injury due to testicular torsion and detorsion.Forty prepubertal albino rats were used. In the IR group, torsion was ... |
|
|
Effects of trapidil on renal ischemia-reperfusion injury.
J. Pediatr. Surg. 41(10) , 1686-93, (2006) There is increasing evidence to suggest that reactive oxygen and nitrogen species play a role in the pathogenesis of renal ischemia-reperfusion (I/R) injury. This study was designed to determine the p... |
|
|
Clinical evidence for Japanese population based on prospective studies—Linking clinical trials and clinical practice
J. Cardiol. 54(2) , 171-82, (2009) “Evidence-based medicine (EBM)” implies effective and high quality practice for patients based on well-grounded medical science. The success of clinical trials in Japan is essential to build original ... |
| Trapidil |
| EINECS 239-434-2 |
| (1,2,4)Triazolo(1,5-a)pyrimidin-7-amine,N,N-diethyl-5-methyl |
| Rocornal |
| 5-methyl-7-diethylamino-s-triazolo[1,5-a]pyrimidine |
| AR 12008,Avantrin,N,N-diethyl-5-methyl-[1,2,4]Triazolo[1,5-a]pyrimidin-7-amine |
| 7-Diethylamino-5-methyl-s-triazolo[1,5-a]pyrimidine |
| N,N-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine |
| N,N-dimethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine |
| diethyl-(5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-amine |
| Trapidilum [INN-Latin] |
| Diaethyl-(5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-amin |
| Trapymin |
| UNII:EYG5Y6355E |
| [1,2,4]Triazolo[1,5-a]pyrimidin-7-amine, N,N-diethyl-5-methyl- |
| 5-Methyl-7-diaethylamino-s-triazolo<1.5-a>pyrimidin |
| N,N-Diethyl-5-methyl[1,2,4]triazolo[1,5-a]pyrimidin-7-amine |
![4-methyl-1,5,7,9-tetrazabicyclo[4.3.0]nona-3,5,7-trien-2-one structure](https://image.chemsrc.com/caspic/303/35523-67-2.png)
![7-Chloro-5-Methyl-[1,2,4]Triazolo[1,5-A]Pyrimidine structure](https://image.chemsrc.com/caspic/395/24415-66-5.png)