Linsidomine hydrochloride

Linsidomine hydrochloride structure
|
Common Name | Linsidomine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 16142-27-1 | Molecular Weight | 206.630 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H11ClN4O2 | Melting Point | 186 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Linsidomine hydrochlorideSIN-1 (chloride) is the active metabolite of molsidomine. SIN-1 (chloride) exhibits potent vasorelaxant effect and inhibition of platelet aggregation[1]. SIN-1 (chloride) decreases myocardial necrosis and reperfusion-induced endothelial dysfunction in models of myocardial ischemia-reperfusion[2]. |
| Name | 3-Morpholinosydnonimine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | SIN-1 (chloride) is the active metabolite of molsidomine. SIN-1 (chloride) exhibits potent vasorelaxant effect and inhibition of platelet aggregation[1]. SIN-1 (chloride) decreases myocardial necrosis and reperfusion-induced endothelial dysfunction in models of myocardial ischemia-reperfusion[2]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 186 °C |
|---|---|
| Molecular Formula | C6H11ClN4O2 |
| Molecular Weight | 206.630 |
| Exact Mass | 206.057053 |
| PSA | 67.28000 |
| Storage condition | -20°C |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent protein kinase.
Cell Death Dis. 5 , e1410, (2014) Platelet activation is a frontline response to injury, not only essential for clot formation but also important for tissue repair. Indeed, the reparative influence of platelets has long been exploited... |
|
|
Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells.
Exp. Neurol. 233(1) , 154-62, (2012) The role of taurine in regulating glucose-induced nitrosative stress has been examined in human Schwann cells, a model for understanding the pathogenesis of diabetic neuropathy. Exposure to high gluco... |
|
|
Comparative kinetics of thiol oxidation in two distinct free-radical generating systems: SIN-1 versus AAPH.
Free Radic. Res. 46(10) , 1190-200, (2012) To study oxidative stress in biological systems, chemical compounds capable of producing free radicals have been widely used. Here, we compared two free-radical generators, 3-morpholinosydnonimine (SI... |
| 3-(4-morpholinyl)-5-sydnone imine hydrochloride |
| [3-(4-Morpholinyl)-1,2,3-oxadiazol-3-ium-5-yl]azanide hydrochloride (1:1) |
| 3-(4-Morpholinyl)sydnone imine hydrochloride Linsidomine hydrochloride SIN-1 hydrochloride |
| T6N DOTJ A- AT5KNOYJ DUM &&sydnone |
| SIN-I HYDROCHLORIDE |
| LINSIDOMINE HCL |
| 3-(4-morpholinyl)-sydnonimine hydrochloride |
| [3-(Morpholin-4-yl)-1,2,3-oxadiazol-3-ium-5-yl]azanide hydrochloride (1:1) |
| MFCD00221615 |
| 3-(4-Morpholinyl)sydnone imine hydrochloride |
| LinsidoMine Chlorhydrate |
| 3-morpholinosydnone imine hydrochloride |
| 1,2,3-Oxadiazolium, 5-amino-3-(4-morpholinyl)-, inner salt, hydrochloride (1:1) |
| MolsidoMine IMpurity A |
| SIN-1 |
| SIN-1 CHLORIDE |
| B-HT 933 dihydrochloride |
| 3-morpholino-sydnonimine hydrochloride |
| SIN-1 HCL |
| 3-Morpholinosydnonimine hydrochloride |
| 5-Imino-3-(morpholin-4-yl)-5H-1,2,3-oxadiazol-3-ium-2-ide hydrochloride (1:1) |
| SIN-1 HYDROCHLORIDE |
| EINECS 247-207-4 |
| LINSIDOMINE |
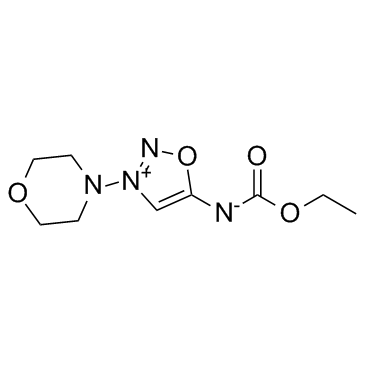 CAS#:25717-80-0
CAS#:25717-80-0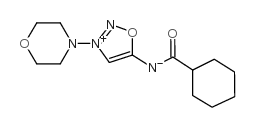 CAS#:66564-16-7
CAS#:66564-16-7