Ethoxymethaniminium chloride
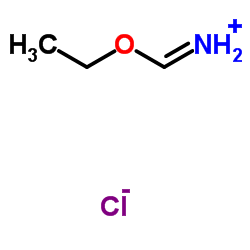
Ethoxymethaniminium chloride structure
|
Common Name | Ethoxymethaniminium chloride | ||
|---|---|---|---|---|
| CAS Number | 16694-46-5 | Molecular Weight | 109.555 | |
| Density | 0.88g/cm3 | Boiling Point | 31.7ºC at 760mmHg | |
| Molecular Formula | C3H8ClNO | Melting Point | 75ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | ethyl methanimidate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Density | 0.88g/cm3 |
|---|---|
| Boiling Point | 31.7ºC at 760mmHg |
| Melting Point | 75ºC |
| Molecular Formula | C3H8ClNO |
| Molecular Weight | 109.555 |
| Exact Mass | 109.029442 |
| PSA | 33.08000 |
| LogP | 1.53170 |
| Vapour Pressure | 597mmHg at 25°C |
| Index of Refraction | 1.388 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2925290090 |
|
~67% 
Ethoxymethanimi... CAS#:16694-46-5 |
| Literature: LEGOCHEM BIOSCIENCE LTD.; GREEN CROSS CORPORATION Patent: US2012/136149 A1, 2012 ; Location in patent: Page/Page column 8 ; |
|
~91% 
Ethoxymethanimi... CAS#:16694-46-5 |
| Literature: Cavalieri, Liebe F.; Tinker, John F.; Bendich, Aaron. Journal of the American Chemical Society, 1949 , vol. 71, # 2 p. 533 - 536 |
|
~%
Detail
|
| Literature: Chemische Berichte, , vol. 16, p. 362 |
| Precursor 5 | |
|---|---|
| DownStream 9 | |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Purines, pyrimidines, and imidazoles. XL. A new synthesis of a D-ribofuranosylamine derivative and its use in the synthesis of pyrimidine and imidazole nucleosides.
J. Chem. Soc. Perkin Trans. I 16 , 1720-31, (1973)
|
|
|
Synthesis of the S-Triazine System. Iii. 1 Trimerization of Imidates. Schaefer FC and Peters GA.
J. Org. Chem. 26(8) , 2771-84, (1961)
|
|
|
Purines, pyrimidines, and imidazoles. Part XLI. Glycofuranosylamines derived from D-xylose, D-glucose, D-mannose, and L-rhamnose and their use in the synthesis of pyrimidine and imidazole nucleosides. Cusack NJ, et al.
J. Chem. Soc. Perkin Trans. I , 73-81, (1974)
|
| Ethoxymethaniminium chloride |
| HCl*HN=CHOEt |
| formimidic acid ethyl ester hydrochloride |
| Methaniminium, 1-ethoxy-, chloride (1:1) |
| ethyl formimidate.HCl |
| O-ethyl formimidate hydrochloride |
| Ethyl formalimidate HCl |
| MFCD00192154 |
| Ethyl formimidate hydrochloride |
| ethyl methanimidate hydrochloride |



 CAS#:93-61-8
CAS#:93-61-8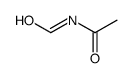 CAS#:21163-79-1
CAS#:21163-79-1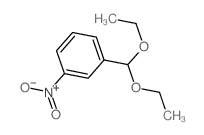 CAS#:2403-49-8
CAS#:2403-49-8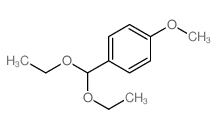 CAS#:2403-58-9
CAS#:2403-58-9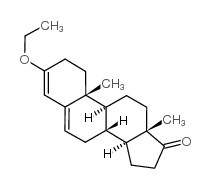 CAS#:972-46-3
CAS#:972-46-3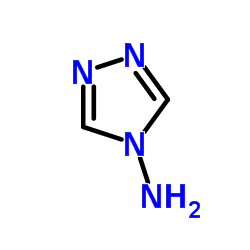 CAS#:584-13-4
CAS#:584-13-4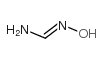 CAS#:624-82-8
CAS#:624-82-8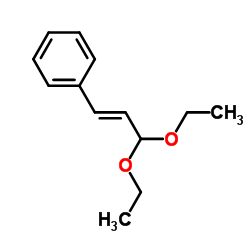 CAS#:7148-78-9
CAS#:7148-78-9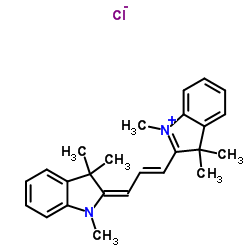 CAS#:6320-14-5
CAS#:6320-14-5