Sodium zirconium cyclosilicate
Modify Date: 2024-01-04 17:46:46
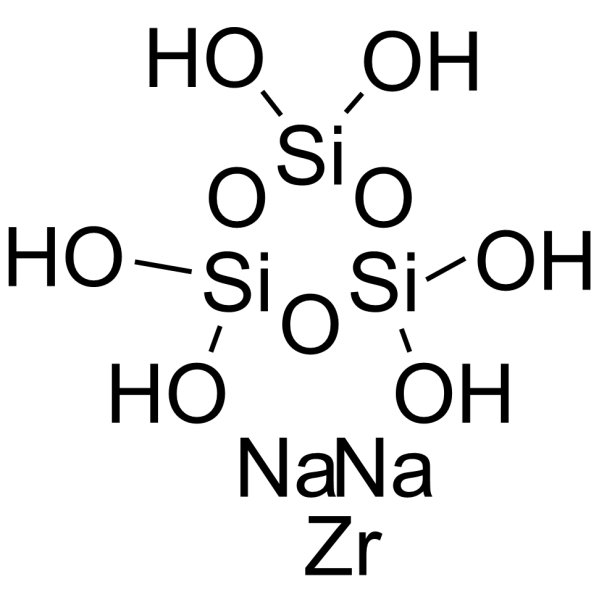
Sodium zirconium cyclosilicate structure
|
Common Name | Sodium zirconium cyclosilicate | ||
|---|---|---|---|---|
| CAS Number | 17141-74-1 | Molecular Weight | 371.50 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | H6O9Si3.2Na.Zr | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Sodium zirconium cyclosilicateSodium zirconium cyclosilicate is an orally administered, non-absorbed, novel, inorganic microporous zirconium silicate compound, is a highly selective cation exchanger that selectively removes excess K+ in vivo. Sodium zirconium cyclosilicate can be used in research of chronic kidney disease (CKD)[1][2]. |
| Name | Sodium zirconium cyclosilicate |
|---|
| Description | Sodium zirconium cyclosilicate is an orally administered, non-absorbed, novel, inorganic microporous zirconium silicate compound, is a highly selective cation exchanger that selectively removes excess K+ in vivo. Sodium zirconium cyclosilicate can be used in research of chronic kidney disease (CKD)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Sodium zirconium cyclosilicate (ZS-9) is an inorganic cation exchange crystalline compound that has a high capacity to selectively entrap monovalent cations, specifically excess K+ and ammonium ions. Sodium zirconium cyclosilicate has the exchange capacities for the divalent ions Ca2+ and Mg2+ are below 0.05 mEq/g, and a >25-fold selectivity for K+ over either Ca2+ or Mg2+[1]. |
| In Vivo | Sodium zirconium cyclosilicate (ZS-9; 2-6 g/kg; p.o.; daily, for 5 d) is a recovery in feces of Sprague-Dawley rats and can efficient uptake and removal of potassium ions[2]. Animal Model: Sprague-Dawley rats[2] Dosage: 2, 4, and 6 g/kg Administration: Oral administration; daily, for 5 days Result: Had 99% fecal recovery in rats. Uptake and removal of potassium ions in a dose-dependent manner. |
| Molecular Formula | H6O9Si3.2Na.Zr |
|---|---|
| Molecular Weight | 371.50 |