γ-Cyclodextrin
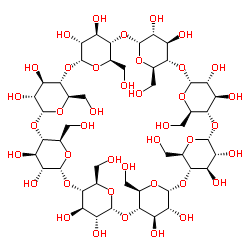
γ-Cyclodextrin structure
|
Common Name | γ-Cyclodextrin | ||
|---|---|---|---|---|
| CAS Number | 17465-86-0 | Molecular Weight | 1297.125 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 845.2°C (rough estimate) | |
| Molecular Formula | C48H80O40 | Melting Point | 267ºC | |
| MSDS | Chinese USA | Flash Point | 450℃ | |
Use of γ-Cyclodextrinγ-Cyclodextrin is an endogenous metabolite. |
| Name | γ-cyclodextrin |
|---|---|
| Synonym | More Synonyms |
| Description | γ-Cyclodextrin is an endogenous metabolite. |
|---|---|
| Related Catalog |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 845.2°C (rough estimate) |
| Melting Point | 267ºC |
| Molecular Formula | C48H80O40 |
| Molecular Weight | 1297.125 |
| Flash Point | 450℃ |
| Exact Mass | 1296.422607 |
| PSA | 633.20000 |
| LogP | -8.97 |
| Index of Refraction | 1.591 |
| Water Solubility | 1 M NaOH: 25 mg/mL, may be clear to slightly hazy |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | GU2293080 |
| HS Code | 3505100000 |
|
~% 
γ-Cyclodextrin CAS#:17465-86-0 |
| Literature: Bioscience, biotechnology, and biochemistry, , vol. 67, # 2 p. 334 - 340 |
|
~%
Detail
|
| Literature: Carbohydrate Research, , vol. 341, # 2 p. 210 - 217 |
|
~% 
γ-Cyclodextrin CAS#:17465-86-0 |
| Literature: Journal of Physical Chemistry B, , vol. 107, # 42 p. 11652 - 11659 |
|
~% 
γ-Cyclodextrin CAS#:17465-86-0 |
| Literature: Chemistry Letters, , p. 1825 - 1828 |
|
~% 
γ-Cyclodextrin CAS#:17465-86-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 29, # 1 p. 213 - 219 |
|
~% 
γ-Cyclodextrin CAS#:17465-86-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 29, # 1 p. 213 - 219 |
| Precursor 5 | |
|---|---|
| DownStream 5 | |
| HS Code | 3505100000 |
|---|
|
Phthalimido-ferrocidiphenol cyclodextrin complexes: Characterization and anticancer activity.
Int. J. Pharm. 491 , 323-34, (2015) Several ferrocenyl analogues of tamoxifen have already showed strong antiproliferative activity in experimental glioma models. Nevertheless, these compounds are very poorly soluble in water and an ada... |
|
|
Effect of γ-cyclodextrin on the in vitro skin permeation of a steroidal drug from nanoemulsions: impact of experimental setup.
Int. J. Pharm. 423(2) , 535-42, (2012) Numerous reports on the enhancement effect of cyclodextrins (CDs) on the skin permeation of dermally applied drugs exist, the majority of which is based on in vitro diffusion cell studies. The specifi... |
|
|
Development of sucrose stearate-based nanoemulsions and optimisation through γ-cyclodextrin.
Eur. J. Pharm. Biopharm. 79(1) , 58-67, (2011) Nanoemulsions aimed at dermal drug delivery are usually stabilised by natural lecithins. However, lecithin has a high tendency towards self-aggregation and is prone to chemical degradation. Therefore,... |
| Cyclooctaamylose |
| MFCD00149574 |
| g-Dextrin |
| RINGDEX C |
| (1S,3R,5R,6S,8R,10R,11S,13R,15R,16S,18R,20R,21S,23R,25R,26S,28R,30R,31S,33R,35R,36S,38R,40R,41R,42R,43R,44R,45R,46R,47R,48R,49R,50R,51R,52R,53R,54R,55R,56R)-5,10,15,20,25,30,35,40-Octakis(hydroxymethyl)-2,4,7,9,12,14,17,19,22,24,27,29,32,34,37,39-hexadecaoxanonacyclo[36.2.2.2.2.2.2.2.2.2]hexapentacontane-41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56-hexadecol (non-preferred name) |
| gamma-cyclodextrin |
| (1S,3R,5R,6S,8R,10R,11S,13R,15R,16S,18R,20R,21S,23R,25R,26S,28R,30R,31S,33R,35R,36S,38R,40R,41R,42R,43R,44R,45R,46R,47R,48R,49R,50R,51R,52R,53R,54R,55R,56R)-5,10,15,20,25,30,35,40-Octakis(hydroxymethy ;l)-2,4,7,9,12,14,17,19,22,24,27,29,32,34,37,39-hexadecaoxanonacyclo[36.2.2.2.2.2.2.2.2.2]hexapentacontane-41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56- hexadecol |
| EINECS 241-482-4 |
| Schardinger γ-Dextrin |
| g-Cyclodextrin |
| γ-Dextrin |
| Cyclooctapentylose |
| γ-Cyclodextrin |
| Dexy Pearl g-100 |
| Cyclomaltooctaose |
| Dexy Pearl γ-100 |
| Sugammadex Impurity 17 |


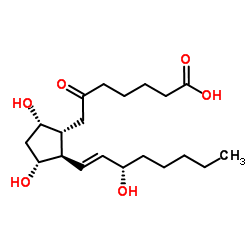
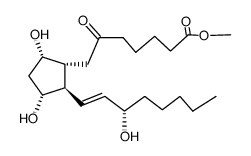
 CAS#:51166-71-3
CAS#:51166-71-3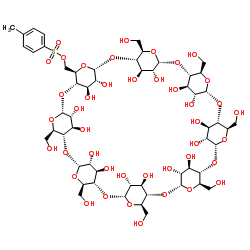 CAS#:97227-33-3
CAS#:97227-33-3 CAS#:97227-32-2
CAS#:97227-32-2 CAS#:129499-78-1
CAS#:129499-78-1
