cPrPMEDAP
Modify Date: 2024-01-14 12:37:03
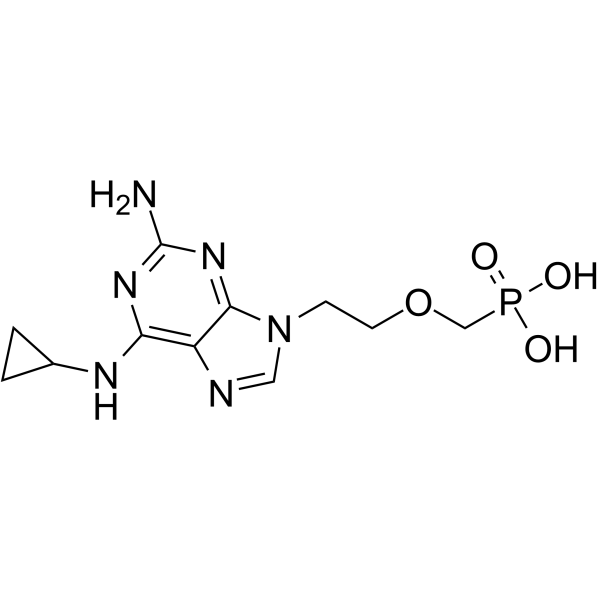
cPrPMEDAP structure
|
Common Name | cPrPMEDAP | ||
|---|---|---|---|---|
| CAS Number | 182798-83-0 | Molecular Weight | 328.26 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H17N6O4P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of cPrPMEDAPcPrPMEDAP is an intermediate metabolite of GS-9219. cpr-PMEDAP functions as a prodrug of the guanine nucleotide analog PMEG and has antiproliferative activity. cPrPMEDAP is negatively charged at physiologic pH and has poor permeability into the skin[1][2]. |
| Name | cPrPMEDAP |
|---|
| Description | cPrPMEDAP is an intermediate metabolite of GS-9219. cpr-PMEDAP functions as a prodrug of the guanine nucleotide analog PMEG and has antiproliferative activity. cPrPMEDAP is negatively charged at physiologic pH and has poor permeability into the skin[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | cPrPMEDAP shows antiproliferative activity in SiHa cells with an EC50 of 290 nM (SiHa cells: HPV-transformed cervical carcinoma cell lines)[1]. PMEG forms an active phosphorylated metabolite, PMEG diphosphate (PMEG-DP), in cells, which inhibits the growth of various transformed cell lines due to potent inhibition of the nuclear DNA polymerases α, δ and ɛ, resulting in inhibition of DNA synthesis and/or DNA repair. In animal models, PMEG has antiproliferative effects; the utility of PMEG as an antiproliferative agent is limited by its poor cellular permeability and toxicity. cPrPMEDAP has similar antiproliferative effects in vitro and reduced toxicity in vivo but, like PMEG, is negatively charged at physiologic pH and has poor permeability into the skin[1]. |
| References |
| Molecular Formula | C11H17N6O4P |
|---|---|
| Molecular Weight | 328.26 |