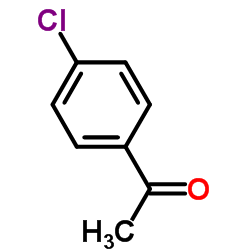
SC-560

SC-560 structure
|
Common Name | SC-560 | ||
|---|---|---|---|---|
| CAS Number | 188817-13-2 | Molecular Weight | 352.738 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 440.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H12ClF3N2O | Melting Point | 63 °C | |
| MSDS | Chinese USA | Flash Point | 220.3±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of SC-560SC-560 is a potent and selective COX-1 inhibitor with an IC50 of 9 nM. |
| Name | 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)pyrazole |
|---|---|
| Synonym | More Synonyms |
| Description | SC-560 is a potent and selective COX-1 inhibitor with an IC50 of 9 nM. |
|---|---|
| Related Catalog | |
| Target |
COX-1:9 nM (IC50) COX-2:6.3 μM (IC50) |
| In Vitro | Preincubation of COX-1 with SC-560 inhibits the conversion of arachidonic acid to PGE2 in a concentration-dependent manner. The IC50 of SC-560 for COX-2 is 6.3 μM, nearly 1,000-fold higher than with COX-1[1]. SC-560 shows a dose and time dependent inhibitory effect on HCC cell growth. SC-560 also inhibits colony formation in soft agar and induces apoptosis in HCC cells in a dose-dependent manner. Moreover, SC-560 decreases the levels of the anti-apoptotic proteins survivin and XIAP and activates caspase 3 and 7 in a dose and time dependent fashion[2]. |
| In Vivo | Oral dosing with either 10 or 30 mg/kg SC-560 1 hour before assay completely inhibits ionophore-stimulated TxB2production, indicating that SC-560 is orally bioavailable and inhibits COX-1 in vivo[1]. SC-560 extensively distributes into rat tissues, and has a CL approaching hepatic plasma flow. The drug displays low less than 15% and formulation dependent bioavailability after oral administration and demonstrates kidney toxicity[3]. |
| Cell Assay | HuH-6 and HA22T/VGH cells (5000/well) are treated with various concentrations of SC-560 (5, 10, 25, 50, 100, 200 μM) and cultured for 72 h. At the end of treatment, cell viability is assessed by MTS assay[2]. |
| Animal Admin | Rats: The pharmacokinetics of SC-560 is studied in Sprague-Dawley rats after a single intravenous (i.v.) and oral dose (10 mg/kg) in polyethylene glycol (PEG) 600 and a single oral dose (10 mg/kg) in 1% methylcellulose (MC). Serial blood samples are collected via a catheter inserted in the right jugular vein and serum samples are analysed for SC-560 using reverse phase HPLC. After oral administration of SC-560 in PEG, urine is also collected for 24 h and analyzed for urinary sodium, chloride, and potassium as well as NAG[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 440.6±45.0 °C at 760 mmHg |
| Melting Point | 63 °C |
| Molecular Formula | C17H12ClF3N2O |
| Molecular Weight | 352.738 |
| Flash Point | 220.3±28.7 °C |
| Exact Mass | 352.059021 |
| PSA | 27.05000 |
| LogP | 6.13 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.564 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933199090 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933199090 |
|---|---|
| Summary | 2933199090. other compounds containing an unfused pyrazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Arachidonic acid downregulates acyl-CoA synthetase 4 expression by promoting its ubiquitination and proteasomal degradation.
J. Lipid Res. 55(8) , 1657-1667, (2014) ACSL4 is a member of the long-chain acyl-CoA synthetase (ACSL) family with a marked preference for arachidonic acid (AA) as its substrate. Although an association between elevated levels of ACSL4 and ... |
|
|
Mechanisms for proteinase-activated receptor 1-triggered prostaglandin E2 generation in mouse osteoblastic MC3T3-E1 cells.
Biol. Chem. 396(2) , 153-62, (2015) We analyzed signaling mechanisms for prostaglandin E2 (PGE2) production following activation of proteinase-activated receptor-1 (PAR1), a thrombin receptor, in preosteoblastic MC3T3-E1 cells. PAR1 sti... |
|
|
COX-2 inhibition improves retinal function in rats' ischemic eyes.
J. Ocul. Pharmacol. Ther. 30(8) , 634-41, (2014) Retinal ischemia is a relatively simple model for studies in pharmacological neuroprotective intervention. The role of cyclooxygenase (COX) enzymes in ischemic insult has been variously shown to eithe... |
| Ionomycin calcium salt |
| Lopac-S-2064 |
| 5-(4-Chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole |
| 1H-Pyrazole, 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)- |
| 5-(4-Chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethyl pyrazole |
| SC560 |
| SC-560 |