2-Bromo-3-nitropyridine
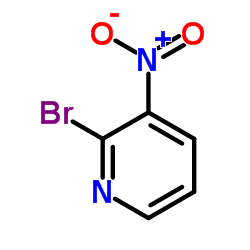
2-Bromo-3-nitropyridine structure
|
Common Name | 2-Bromo-3-nitropyridine | ||
|---|---|---|---|---|
| CAS Number | 19755-53-4 | Molecular Weight | 202.993 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 244.0±20.0 °C at 760 mmHg | |
| Molecular Formula | C5H3BrN2O2 | Melting Point | 122-125 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 101.4±21.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Bromo-3-nitropyridine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 244.0±20.0 °C at 760 mmHg |
| Melting Point | 122-125 °C(lit.) |
| Molecular Formula | C5H3BrN2O2 |
| Molecular Weight | 202.993 |
| Flash Point | 101.4±21.8 °C |
| Exact Mass | 201.937775 |
| PSA | 58.71000 |
| LogP | 1.17 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.614 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933399090 |
|
~% 
2-Bromo-3-nitro... CAS#:19755-53-4 |
| Literature: Journal of the Chemical Society, , p. 2042,2044 |
|
~% 
2-Bromo-3-nitro... CAS#:19755-53-4
Detail
|
| Literature: Journal of Organic Chemistry, , vol. 77, # 9 p. 4402 - 4413 |
|
~46% 
2-Bromo-3-nitro... CAS#:19755-53-4 |
| Literature: Loidreau, Yvonnick; Marchand, Pascal; Dubouilh-Benard, Carole; Nourrisson, Marie-Renee; Duflos, Muriel; Lozach, Olivier; Loaec, Nadege; Meijer, Laurent; Besson, Thierry European Journal of Medicinal Chemistry, 2012 , vol. 58, p. 171 - 183 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Furanylazaindoles: potent anticancer agents in vitro and in vivo.
J. Med. Chem. 56(20) , 8008-18, (2013) Preliminary biological data on 7-anilino-6-azaindoles (8-11) suggested that hydrophobic substituents at C7 contribute to enhancement of antiproliferative activity. A novel series of 7-aryl-6-azaindole... |
|
|
Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors.
Eur. J. Med. Chem. 58 , 171-83, (2012) A useful and rapid access to libraries of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues was designed and optimized for the first time via microwave-accelerated ... |
|
|
Condensed heteroaromatic ring systems. XII. Synthesis of indole derivatives from ethyl 2-bromocarbanilates. Sakamoto T, et al.
Chem. Pharm. Bull. 35(5) , 1823-1828, (1987)
|
| MFCD00955613 |
| 3-Nitro-2-bromopyridine |
| 2-Brom-3-nitro-pyridin |
| Pyridine, 2-bromo-3-nitro- |
| Brom-2-nitro-3-pyridin |
| 2-bromo-3-nitro-pyridine |
| 2-Bromo-3-nitropyridine |
| 2-bromonitropyridine |
| Pyridine,2-bromo-3-nitro |
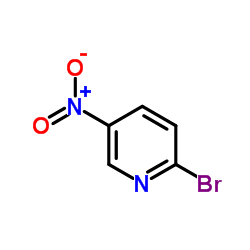
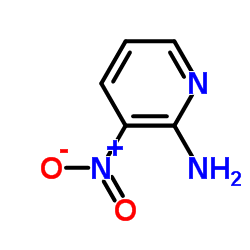
 CAS#:42242-11-5
CAS#:42242-11-5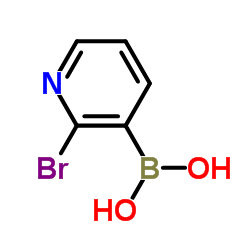 CAS#:452972-08-6
CAS#:452972-08-6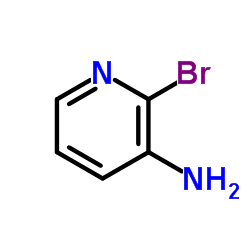 CAS#:39856-58-1
CAS#:39856-58-1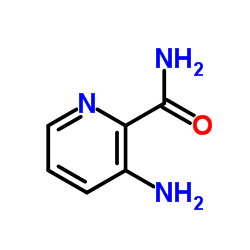 CAS#:50608-99-6
CAS#:50608-99-6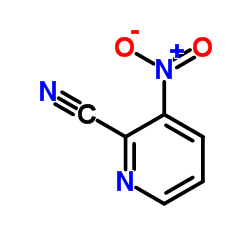 CAS#:51315-07-2
CAS#:51315-07-2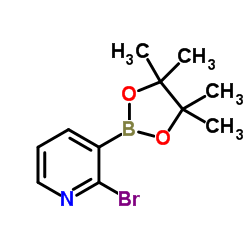 CAS#:452972-12-2
CAS#:452972-12-2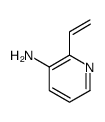 CAS#:267875-96-7
CAS#:267875-96-7 CAS#:272-49-1
CAS#:272-49-1 CAS#:6298-19-7
CAS#:6298-19-7 CAS#:34949-41-2
CAS#:34949-41-2
