benzo[b]naphtho[1,2-d]thiophene
![benzo[b]naphtho[1,2-d]thiophene Structure](https://image.chemsrc.com/caspic/302/205-43-6.png)
benzo[b]naphtho[1,2-d]thiophene structure
|
Common Name | benzo[b]naphtho[1,2-d]thiophene | ||
|---|---|---|---|---|
| CAS Number | 205-43-6 | Molecular Weight | 234.316 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 434.3±14.0 °C at 760 mmHg | |
| Molecular Formula | C16H10S | Melting Point | 185℃ (acetic acid ) | |
| MSDS | N/A | Flash Point | 163.0±6.3 °C | |
| Name | naphtho[2,1-b][1]benzothiole |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 434.3±14.0 °C at 760 mmHg |
| Melting Point | 185℃ (acetic acid ) |
| Molecular Formula | C16H10S |
| Molecular Weight | 234.316 |
| Flash Point | 163.0±6.3 °C |
| Exact Mass | 234.050323 |
| PSA | 28.24000 |
| LogP | 5.61 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.810 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| RTECS | DI2340000 |
|---|---|
| HS Code | 2934999090 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The predominant role of S-oxidation in rat liver metabolism of thiaarenes.
Cancer Lett. 32(1) , 107-16, (1986) Thiaarenes are metabolized by liver microsomes of untreated rats predominantly to sulfones and sulfoxides. After pretreatment of rats with monooxygenase inducers, ring oxidation of thiaarenes is also ... |
|
|
Towards β-selectivity in functional estrogen receptor antagonists.
Org. Biomol. Chem. 10(36) , 7334-46, (2012) Based on the benzo[b]naphtho[1,2-d]furan and benzo[b]naphtho[1,2-d]thiophene frameworks, a series of ligands with different basic side chains (BSCs) has been synthesized and pharmacologically evaluate... |
| Benzo[b]naphtho[1,2-d]thiophene |
| Naphtho[2,1-b]thianaphthene |
| BCR137R_FLUKA |
| benzo<b>naphtho<1,2-b>thiophene |
| Benzo<b>naphthto<1,2-d>thiophene |
| Benzo[a]dibenzothiophene |
| BENZO(B)NAPHTHO(1,2-D)THIOPHENE |
![3-[1-(2-fluorophenyl)naphthalen-2-ylsulfanyl]propionic acid ethyl ester Structure](https://image.chemsrc.com/caspic/466/1264712-19-7.png) CAS#:1264712-19-7
CAS#:1264712-19-7![naphtho[2,1-b][1]benzothiole 7,7-dioxide Structure](https://image.chemsrc.com/caspic/169/20841-53-6.png) CAS#:20841-53-6
CAS#:20841-53-6![2,3,5,6-tetrahydronaphtho[2,1-b]benzo[b]thiophen-4(1H)-one Structure](https://image.chemsrc.com/caspic/270/354816-86-7.png) CAS#:354816-86-7
CAS#:354816-86-7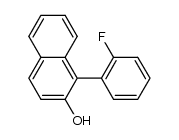 CAS#:1264712-17-5
CAS#:1264712-17-5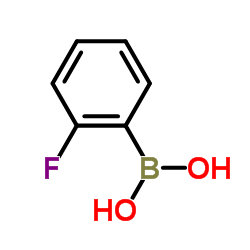 CAS#:1993-03-9
CAS#:1993-03-9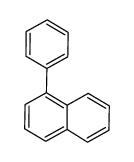 CAS#:605-02-7
CAS#:605-02-7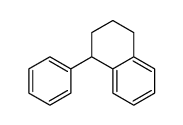 CAS#:3018-20-0
CAS#:3018-20-0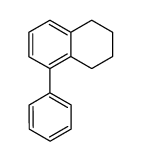 CAS#:67064-63-5
CAS#:67064-63-5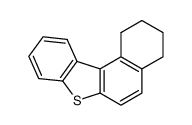 CAS#:90501-14-7
CAS#:90501-14-7
