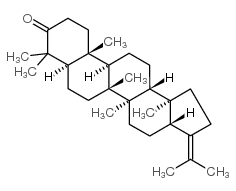
17beta(h),21beta(h)-hop-22(29)-en-3-one
Modify Date: 2024-01-05 18:19:14

17beta(h),21beta(h)-hop-22(29)-en-3-one structure
|
Common Name | 17beta(h),21beta(h)-hop-22(29)-en-3-one | ||
|---|---|---|---|---|
| CAS Number | 25615-11-6 | Molecular Weight | 424.70200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C30H48O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of 17beta(h),21beta(h)-hop-22(29)-en-3-one3-Oxo-hop-22(29)-ene is a yeast α-glucosidase inhibitor. 3-Oxo-hop-22(29)-ene shows a moderate effect on the viability of T. cruzi and L. mexicana. 3-Oxo-hop-22(29)-ene shows marginal activity of anti-inflammatory[1]. |
| Name | 17beta(h),21beta(h)-hop-22(29)-en-3-one |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Oxo-hop-22(29)-ene is a yeast α-glucosidase inhibitor. 3-Oxo-hop-22(29)-ene shows a moderate effect on the viability of T. cruzi and L. mexicana. 3-Oxo-hop-22(29)-ene shows marginal activity of anti-inflammatory[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 3-Oxo-hop-22(29)-ene (compound 2) (50 μM; 24 hours) reduces the viability of T. cruzi by more than 20%, and has a moderate effecton on T. rangeli and L. mexicana[1]. 3-Oxo-hop-22(29)-ene (10, 100 μM; 48 hours) causes 1.51% and 7.39% inhibition activity to yeast α-glucosidase respectively[1]. Cell Viability Assay[1] Cell Line: Saccharomyces cerevisiae (type 1) and mammalian yeast (type 2) α-glucosidases, T. cruzi, T. rangeli, L. mexicana Concentration: 10 μM, 50 μM, 100 μM Incubation Time: 24 hours, 48 hours Result: Reduced the viability of T. cruzi by more than 20%, and had a moderate effecton on T. rangeli and L. mexicana Inhibited Yeast α-glucosidase. |
| In Vivo | 3-Oxo-hop-22(29)-ene (0.31 μmol/ear; application; once) decreases 17.50 % the inflammation of mouse ear edema model[1]. Animal Model: Adult male CD-1 mice (25-30 g) (Mouse ear edema model induced by 12-O-tetradecanoylphorbol acetate (TPA))[1] Dosage: 0.31 μmol/ear Administration: Apply to both faces of the right ear; once Result: Decreased 17.50% the inflammation of mouse ear edema model. |
| References |
| Molecular Formula | C30H48O |
|---|---|
| Molecular Weight | 424.70200 |
| Exact Mass | 424.37100 |
| PSA | 17.07000 |
| LogP | 8.23300 |
|
~% 
17beta(h),21bet... CAS#:25615-11-6 |
| Literature: Murakami,T.; Chen,C.-M. Chemical and Pharmaceutical Bulletin, 1971 , vol. 19, p. 25 - 30 |
|
~% 
17beta(h),21bet... CAS#:25615-11-6 |
| Literature: Aimi, Norio; Kawada, Kazuhiro; Sakai, Shin-ichiro Chemical and Pharmaceutical Bulletin, 1983 , vol. 31, # 10 p. 3765 - 3768 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| androsteronehemisuccinate |

 CAS#:28196-47-6
CAS#:28196-47-6