H-Phe-Phe-OH
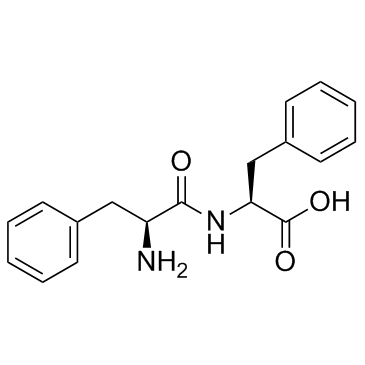
H-Phe-Phe-OH structure
|
Common Name | H-Phe-Phe-OH | ||
|---|---|---|---|---|
| CAS Number | 2577-40-4 | Molecular Weight | 312.363 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 582.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H20N2O3 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 306.2±30.1 °C | |
Use of H-Phe-Phe-OHH-Phe-Phe-OH is a peptide made of two phenylalanine molecules; Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine. |
| Name | Phe-Phe |
|---|---|
| Synonym | More Synonyms |
| Description | H-Phe-Phe-OH is a peptide made of two phenylalanine molecules; Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | H-Phe-Phe-OH (Phe-Phe) is a peptide made of two phenylalanine molecules. Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine. It is the precursor of catecholamines in the body. The psychotropic drugs (mescaline, morphine, codeine, and papaverine) also have phenylalanine as a constituent. The H-Phe-Phe-OH recognition motif of the Alzheimer's Abeta peptide is the smallest peptide able to assemble into higher -order structures[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.8±50.0 °C at 760 mmHg |
| Molecular Formula | C18H20N2O3 |
| Molecular Weight | 312.363 |
| Flash Point | 306.2±30.1 °C |
| Exact Mass | 312.147400 |
| PSA | 92.42000 |
| LogP | 2.48 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.605 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3.0 |
| HS Code | 2924299090 |
| Precursor 9 | |
|---|---|
| DownStream 5 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Metabolomic profiling of serum in the progression of Alzheimer's disease by capillary electrophoresis-mass spectrometry.
Electrophoresis 35(23) , 3321-30, (2014) There is high interest in the discovery of early diagnostic biomarkers of Alzheimer's disease, for which metabolomics exhibits a great potential. In this work, a metabolomic approach based on ultrafil... |
|
|
Possible mechanism of uptake for several compounds in ionized form through human erythrocyte membrane.
Life Sci. 34(5) , 427-36, (1984) The mechanism underlying uptake of certain compounds in ionized form across human red blood cell membrane was examined. The ionized forms of salicylate, 5-methoxy-salicylate and phenylalanylphenylalan... |
|
|
pH-dependence of complexion constants and complex mobility in capillary electrophoresis separations of dipeptide enantiomers.
Electrophoresis 22(15) , 3163-70, (2001) The chiral separation of the LL- and DD-enantiomers of the dipeptides Ala-Tyr, Phe-Phe, and Asp-PheOMe has been investigated at pH 2.5 and pH 3.5 using beta-cyclodextrin (beta-CD), heptakis-(2,6-di-O-... |
| L-Phe-L-Phe-OH |
| Phe-Phe |
| l-Phe-l-Phe |
| L-Phenylalanyl-L-phenylalanine |
| (S)-2-((S)-2-Amino-3-phenylpropanamido)-3-phenylpropanoic acid |
| L-Phenylalanyl-L-phenylalanine Di-L-phenylalanine |
| Di-L-phenylalanine |
| Phenylalanyl-phenylalanine |
| L-Phenylalanine, L-phenylalanyl- |
| 2-[(2-amino-3-phenylpropanoyl)amino]-3-phenylpropanoic acid |
| H-Phe-Phe-OH |
 CAS#:63-91-2
CAS#:63-91-2 CAS#:35661-40-6
CAS#:35661-40-6 CAS#:5241-58-7
CAS#:5241-58-7 CAS#:2577-90-4
CAS#:2577-90-4 CAS#:70669-39-5
CAS#:70669-39-5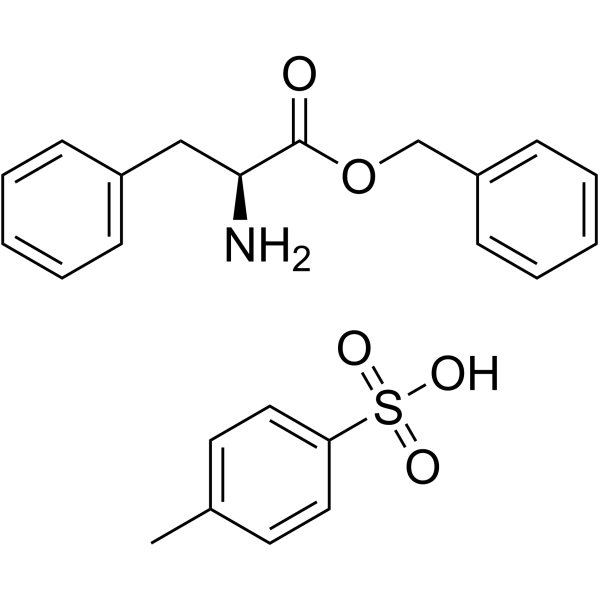 CAS#:1738-78-9
CAS#:1738-78-9 CAS#:14825-82-2
CAS#:14825-82-2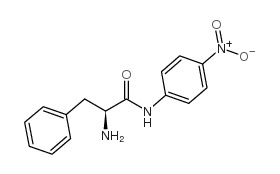 CAS#:2360-97-6
CAS#:2360-97-6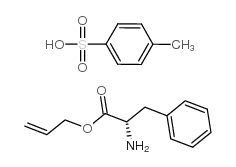 CAS#:88224-00-4
CAS#:88224-00-4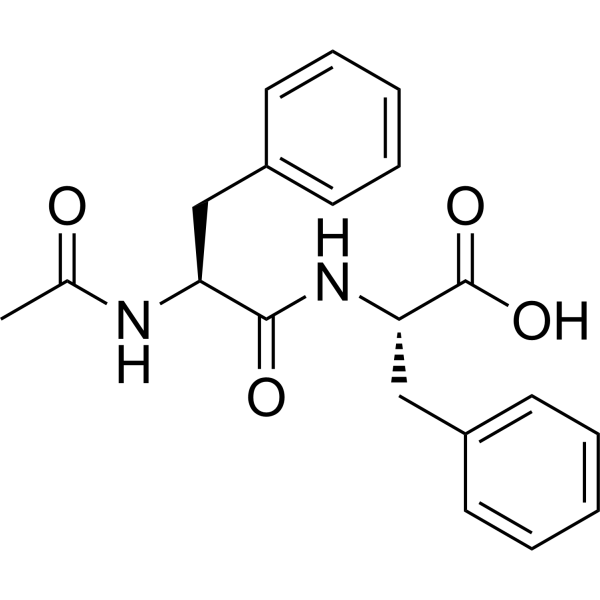 CAS#:10030-31-6
CAS#:10030-31-6 CAS#:13122-90-2
CAS#:13122-90-2 CAS#:13122-91-3
CAS#:13122-91-3 CAS#:38017-65-1
CAS#:38017-65-1
