Oridonin
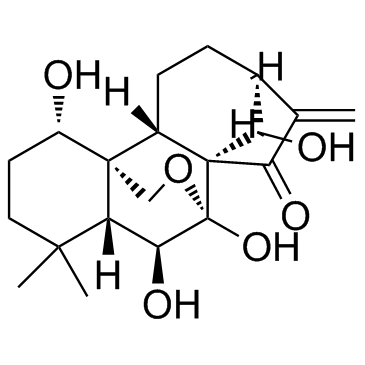
Oridonin structure
|
Common Name | Oridonin | ||
|---|---|---|---|---|
| CAS Number | 28957-04-2 | Molecular Weight | 364.433 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 599.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H28O6 | Melting Point | 248-250ºC | |
| MSDS | Chinese USA | Flash Point | 215.0±23.6 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of OridoninOridonin, a diterpenoid isolated from Rabdosia rubescens, acts as an inhibitor of AKT, with IC50s of 8.4 and 8.9 μM for AKT1 and AKT2; Oridonin possesses anti-tumor, anti-bacterial and anti-inflammatory effects. |
| Name | Kaur-16-en-15-one, 7,20-epoxy-1,6,7,14-tetrahydroxy-, (1a,6b,7a,14R) |
|---|---|
| Synonym | More Synonyms |
| Description | Oridonin, a diterpenoid isolated from Rabdosia rubescens, acts as an inhibitor of AKT, with IC50s of 8.4 and 8.9 μM for AKT1 and AKT2; Oridonin possesses anti-tumor, anti-bacterial and anti-inflammatory effects. |
|---|---|
| Related Catalog | |
| Target |
Akt1:8.4 μM (IC50) Akt2:8.9 μM (IC50) |
| In Vitro | Oridonin is an ATP-competitive inhibitor of AKT with IC50s of 8.4 and 8.9 μM for AKT1 and AKT2, respectively. Oridonin (5, 10 or 20 μM) obviously inhibits the growth of KYSE70, KYSE410 and KYSE450 ESCC cells via targeting AKT1/2. Oridonin (10 or 20 μM) causes G2/M phase cell cycle arrest in KYSE70, KYSE410 and KYSE450 cells, and induces apoptosis in these three cell lines at 20 μM. In addition, Oridonin (5, 10 or 20 μM) in combination with cisplatin or 5-FU enhances the inhibition of esophageal squamous cell carcinoma (ESCC) cell growth[1]. Oridonin (0.1 and 1 μM) preferentially suppresses AKT/mTOR signaling. Oridonin (1 μM) also selectively suppresses growth of breast cancer cells with hyperactivation of AKT signaling[2]. |
| In Vivo | Oridonin (160 mg/kg, p.o.) shows significant reduction in the tumor growth without obvious weigh loss in SCID mice bearing EG9 and HEG18 tumor cells. Oridonin treatment also suppresses the expression of Ki-67, phosphorylated AKT, GSK-3β or mTOR in mice[1]. Oridonin (15 mg/kg, i.p.) impairs cell growth in breast cancer with hyperactivation of AKT signaling in nude mice[2]. |
| Kinase Assay | For the AKT kinase assay, the ADP-Glo™ Kinase Assay Kit is used. Active AKT1 or AKT2 kinase and inactive GSK-3β as substrate are mixed by 1× reaction buffer and then added to a white 96-well plate. Pure ATP provided in the kit is serially diluted obtain a final concentration of 0, 1, 10, 50, and 100 μM. GSK-3β is added to reach a final concentration of 2.5, 5, 10 or 20 μM and DMSO is used as a control. The mixed solution is incubated at room temperature and luciferase activity is measured using the Luminoskan Ascent plate reader[1]. |
| Cell Assay | Cells are seeded (6×103 cells/well for KYSE70; 2.5×103 cells/well for KYSE410; 2×103 cells/well for KYSE450) in 96-well plates and incubated for 24 h and then treated with different amounts of Oridonin or vehicle. After incubation for 24, 48 or 72 h, cell proliferation is measured by the MTT assay. For anchorage-independent cell growth assessment, cells (2.5, 5 or 10 μM Oridonin) suspended in complete medium are added to 0.3% agar with vehicle, 2.5, 5 or 10 μM Oridonin in a top layer over a base layer of 0.5% agar with vehicle, 2.5, 5 or 10 μM Oridonin. The cultures are maintained at 37°C in a 5% CO2 incubator for 3 weeks and then colonies are visualized under a microscope and counted using the Image-Pro Plus software program[1]. |
| Animal Admin | Mice[2] Breast cancer cells are harvested and resuspended in 40% Matrigel-Basement Membrane Matrix, LDEV-free, and then injected (100 μL per site) into the fourth pair of mammary fat pads of nude mice (CrTac: NCr-Foxn1nu). Tumors are measured in two dimensions using manual calipers. Tumor volume is calculated using the formula: Volume = 0.5 × length × width × width. Tumor volume is measured every 2-3 days. Upon harvesting, tumors are fixed in formalin overnight and then in 70% ethanol for histopathology analysis. Mice are treated with Oridonin (15 mg/kg) in 1% Pluronic F68 or vehicle (1% Pluronic F68) daily by intraperitoneal (IP) injection. BEZ235 is reconstituted 1:9 in 1-methyl-2 pyrolidone and polyethylene glycol 300 (PEG300) Mice are treated with this compound formulation at 45 mg/kg daily (QD) by oral gavage[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 599.8±50.0 °C at 760 mmHg |
| Melting Point | 248-250ºC |
| Molecular Formula | C20H28O6 |
| Molecular Weight | 364.433 |
| Flash Point | 215.0±23.6 °C |
| Exact Mass | 364.188599 |
| PSA | 107.22000 |
| LogP | 1.44 |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.635 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >20mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| HS Code | 2942000000 |
|---|
|
[Anti-tumor effect of tanshinone II A, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines].
Sichuan Da Xue Xue Bao. Yi Xue Ban 43(3) , 362-6, (2012) To study the anti-tumor effect of tanshinon II A, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines SUP-B15, K562, CEM, HL-60 and NB4.To study the anti-tumor effect of tansh... |
|
|
Induction of human CYP3A4 by huperzine A, ligustrazine and oridonin through pregnane X receptor-mediated pathways.
Pharmazie 69(7) , 532-6, (2014) The pregnane X receptor (PXR) is a key regulator of CYP3A4, which is involved in catalyzing the metabolic conversion of a number of endogenous substrates. In this study, we screened 22 compounds isola... |
|
|
The anticancer effect of oridonin is mediated by fatty acid synthase suppression in human colorectal cancer cells.
J. Gastroenterol. 48(2) , 182-92, (2013) Fatty acid synthase (FAS) inhibitors could be a therapeutic target in cancer treatment. However, only a few FAS inhibitors showing clinical potential have been reported. Oridonin is a diterpenoid isol... |
| ORIDONIN |
| Rubescensin |
| ISODONAL |
| (1α,5β,6β,7β,8α,9β,10α,13α,14R)-1,6,7,14-Tetrahydroxy-7,20-epoxykaur-16-en-15-one |
| RUBESCENSIN A |
| 7a,20-Epoxy-1a,6b,7,14-tetrahydroxy-Kaur-16-en-15-one Isodonol |
| Isodonol |
| megathyrin A |
| Rabdosia rubescens |
 CAS#:81078-03-7
CAS#:81078-03-7 CAS#:81078-04-8
CAS#:81078-04-8![Kaur-16-en-15-one,7,20-epoxy-1,6,7-trihydroxy-14-[(1-oxodecyl)oxy]-, (1a,6b,7a,14R)- (9CI) structure](https://image.chemsrc.com/caspic/454/81078-05-9.png) CAS#:81078-05-9
CAS#:81078-05-9![Kaur-16-en-15-one,7,20-epoxy-1,6,7-trihydroxy-14-[(1-oxododecyl)oxy]-, (1a,6b,7a,14R)- (9CI) structure](https://image.chemsrc.com/caspic/296/79729-72-9.png) CAS#:79729-72-9
CAS#:79729-72-9 CAS#:79729-73-0
CAS#:79729-73-0 CAS#:79729-74-1
CAS#:79729-74-1 CAS#:79729-75-2
CAS#:79729-75-2
