1H-Imidazole-4-carbaldehyde

1H-Imidazole-4-carbaldehyde structure
|
Common Name | 1H-Imidazole-4-carbaldehyde | ||
|---|---|---|---|---|
| CAS Number | 3034-50-2 | Molecular Weight | 96.09 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 367.8±15.0 °C at 760 mmHg | |
| Molecular Formula | C4H4N2O | Melting Point | 174-177 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 179.8±26.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1H-Imidazole-4-carbaldehyde1H-Imidazole-5-carboxaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 1H-imidazole-5-carbaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | 1H-Imidazole-5-carboxaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 367.8±15.0 °C at 760 mmHg |
| Melting Point | 174-177 °C(lit.) |
| Molecular Formula | C4H4N2O |
| Molecular Weight | 96.09 |
| Flash Point | 179.8±26.8 °C |
| Exact Mass | 96.032364 |
| PSA | 45.75000 |
| LogP | 0.20 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.620 |
| Storage condition | Room temperature. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933290090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Endosomal pH responsive polymers for efficient cancer targeted gene therapy.
Colloids Surf. B Biointerfaces 119 , 55-65, (2014) Treatment of human diseases at gene level is always limited by effective gene delivery vectors. In this study, we designed and developed an endosomal pH sensitive targeted gene delivery system, folic ... |
|
|
Convenient synthesis of alkyl esters of urocanic acid.
J. Pharm. Sci. 70(1) , 98-9, (1981) Ethyl, n-dodecyl, and n-hexadecyl esters of urocanic acid (4-imidazoleacrylic acid) were prepared from 4-imidazolecarboxaldehyde in satisfactory yields via the Wittig reaction. |
|
|
Anion sensing and F(-)-induced reversible photoreaction of D-π-A type dye containing imidazole moiety as donor.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 117 , 810-3, (2014) A new donor-π-acceptor (D-π-A) type dye was synthesized by the condensation reaction between 2-cyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran and 4-imidazolecarboxaldehyde. The chemical struc... |
| 4-Imidazolecarboxaldehyde |
| 1H-Imidazole-4-carboxaldehyde |
| 4-Formylimidazole |
| Imidazole-4-carboxaldehyde |
| MFCD00173726 |
| Imidazole-4-carbaldehyde |
| 1H-imidazol-4-carboxaldehyde |
| EINECS 221-227-3 |
| 4-imidazolecarbaldehyde |
| 4-Formyl-1H-imidazole |
| 1H-Imidazole-4-carbaldehyde |
| 4-Acetlimidazole |
| 4(5)-imidazole carboxaldehyde |
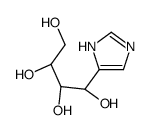 CAS#:2644-71-5
CAS#:2644-71-5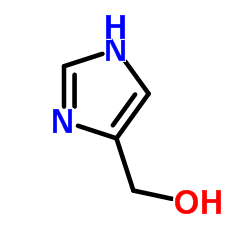 CAS#:822-55-9
CAS#:822-55-9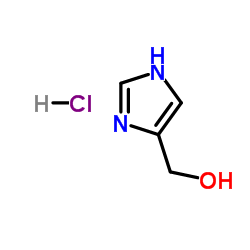 CAS#:32673-41-9
CAS#:32673-41-9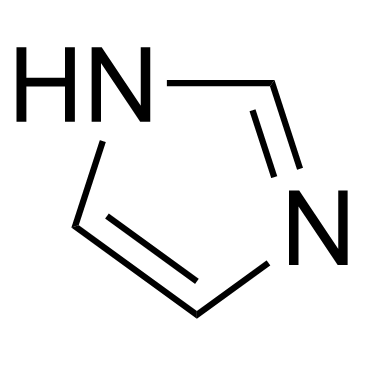 CAS#:288-32-4
CAS#:288-32-4 CAS#:33016-47-6
CAS#:33016-47-6 CAS#:1025907-10-1
CAS#:1025907-10-1 CAS#:3465-72-3
CAS#:3465-72-3 CAS#:340699-23-2
CAS#:340699-23-2 CAS#:39021-62-0
CAS#:39021-62-0 CAS#:17289-26-8
CAS#:17289-26-8 CAS#:57090-88-7
CAS#:57090-88-7 CAS#:85102-93-8
CAS#:85102-93-8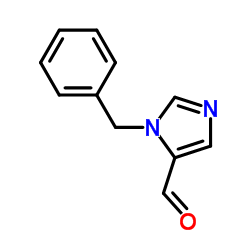 CAS#:85102-99-4
CAS#:85102-99-4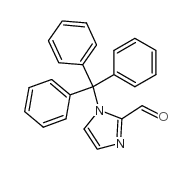 CAS#:67478-50-6
CAS#:67478-50-6 CAS#:15813-09-9
CAS#:15813-09-9 CAS#:199192-04-6
CAS#:199192-04-6
