Panepoxydone
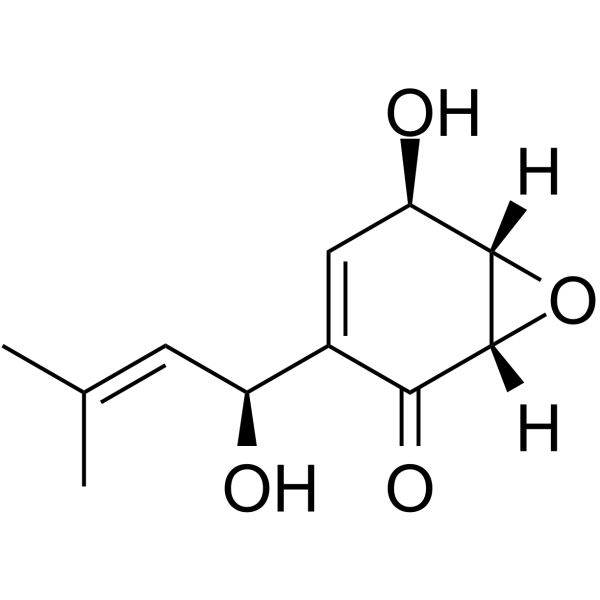
Panepoxydone structure
|
Common Name | Panepoxydone | ||
|---|---|---|---|---|
| CAS Number | 31298-54-1 | Molecular Weight | 210.22600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H14O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PanepoxydonePanepoxydone is an inhibitor of NF-κB activation. Panepoxydone interferes with the NF-κB mediated signal transduction by inhibiting the phosphorylation of IκB. Panepoxydone exhibits antitumor, anti-inflammatory, antimalarial and anti-parasitic activity[1][2]. |
| Name | (1S,2R,6S)-2-hydroxy-4-(1-hydroxy-3-methylbut-2-enyl)-7-oxabicyclo[4.1.0]hept-3-en-5-one |
|---|---|
| Synonym | More Synonyms |
| Description | Panepoxydone is an inhibitor of NF-κB activation. Panepoxydone interferes with the NF-κB mediated signal transduction by inhibiting the phosphorylation of IκB. Panepoxydone exhibits antitumor, anti-inflammatory, antimalarial and anti-parasitic activity[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C11H14O4 |
|---|---|
| Molecular Weight | 210.22600 |
| Exact Mass | 210.08900 |
| PSA | 70.06000 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |
|
Tumor necrosis factor (TNF)-α upregulates progesterone receptor-A by activating the NF-κB signaling pathway in human decidua after labor onset.
Placenta 33(1) , 1-7, (2012) To date, the relationship between inflammatory cytokines and progesterone receptors (PRs) has been little studied, although both mediate the mechanism of parturition in human deciduas. Thus, the aim o... |
|
|
Inhibitors of NF-kappaB signaling: design and synthesis of a biotinylated isopanepoxydone affinity reagent.
Bioorg. Med. Chem. Lett. 12(23) , 3463-6, (2002) A number of inhibitors of NF-kappaB signaling arising from our recent syntheses of isopanepoxydone and panepoxydone have been identified. Structure-activity data have been correlated to allow the desi... |
|
|
In vitro activity of hypnophilin from Lentinus strigosus: a potential prototype for Chagas disease and leishmaniasis chemotherapy.
Braz. J. Med. Biol. Res. 43(11) , 1054-61, (2010) Hypnophilin and panepoxydone, terpenoids isolated from Lentinus strigosus, have significant inhibitory activity on Trypanosoma cruzi trypanothione reductase (TR). Although they have similar TR inhibit... |
| SL-3042 |
| (1s,5r,6s)-5-hydroxy-3-(1-hydroxy-3-methylbut-2-en-1-yl)-7-oxabicyclo[4.1.0]hept-3-en-2-one |
| Antibiotic SL-3042 |
| Panepoxydone |