5-Hydroxy-1-indanone
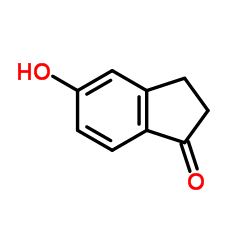
5-Hydroxy-1-indanone structure
|
Common Name | 5-Hydroxy-1-indanone | ||
|---|---|---|---|---|
| CAS Number | 3470-49-3 | Molecular Weight | 148.159 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 343.8±31.0 °C at 760 mmHg | |
| Molecular Formula | C9H8O2 | Melting Point | 175 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 146.4±17.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 5-hydroxy-2,3-dihydroinden-1-one |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 343.8±31.0 °C at 760 mmHg |
| Melting Point | 175 °C (dec.)(lit.) |
| Molecular Formula | C9H8O2 |
| Molecular Weight | 148.159 |
| Flash Point | 146.4±17.4 °C |
| Exact Mass | 148.052429 |
| PSA | 37.30000 |
| LogP | 1.86 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.631 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914400090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914400090 |
|---|---|
| Summary | 2914400090 other ketone-alcohols and ketone-aldehydes。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Synthesis and inhibition study of monoamine oxidase, indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by 3,8-substituted 5H-indeno[1,2-c]pyridazin-5-one derivatives.
Eur. J. Med. Chem. 46(12) , 6104-11, (2011) Previous studies on 5H-indeno[1,2-c]pyridazin-5-one derivatives as inhibitors of MAO-B revealed that it was possible to increase the MAO-B inhibitory potency of 5H-indeno[1,2-c]pyridazin-5-ones by sub... |
|
|
On the involvement of single-bond rotation in the primary photochemistry of photoactive yellow protein. Stahl AD, et al.
Biophys. J. 101(5) , 1184-1192, (2011)
|
|
|
Geometric and solvent effects on intramolecular phenolic hydrogen abstraction by carbonyl n,p* and p,p* triplets. Lathioor EC and Leigh WJ.
Can. J. Chem. 79(12) , 1851-1863, (2001)
|
| 5-Hydroxy-2,3-dihydro-1H-inden-1-on |
| 5-hydroxy-2,3-dihydro-1H-inden-1-one |
| 1H-Inden-1-one, 2,3-dihydro-4-hydroxy- |
| 4-Hydroxy-1-indanone |
| 5-Hydroxy-1-indanone |
| MFCD00857527 |
| 5-hydroxy-2,3-dihydro-1H-indene-1-one |
| 5-hydroxy-indan-1-one |
| 4-hydroxyindan-1-one |
| 5-hydroxy-1-oxo-2,3-dihydro-1H-indene |
| 5-hydroxyindan-1-one |
| 5-Hydroxyindanone |
| 4-hydroxy-2,3-dihydro-1H-inden-1-one |
| 1-oxo-5-indanol |
| 1H-Inden-1-one, 2,3-dihydro-5-hydroxy- |
| 4-Hydroxy-2,3-dihydro-1H-inden-1-on |
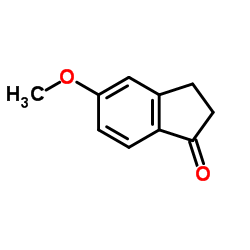 CAS#:5111-70-6
CAS#:5111-70-6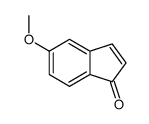 CAS#:72913-59-8
CAS#:72913-59-8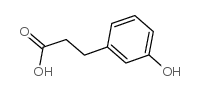 CAS#:621-54-5
CAS#:621-54-5 CAS#:19576-08-0
CAS#:19576-08-0 CAS#:41877-16-1
CAS#:41877-16-1 CAS#:875-59-2
CAS#:875-59-2 CAS#:2373-80-0
CAS#:2373-80-0 CAS#:14755-02-3
CAS#:14755-02-3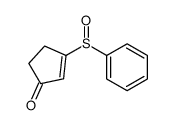 CAS#:64299-69-0
CAS#:64299-69-0 CAS#:59414-23-2
CAS#:59414-23-2 CAS#:105920-67-0
CAS#:105920-67-0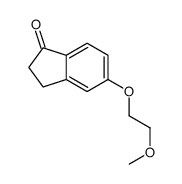 CAS#:28945-97-3
CAS#:28945-97-3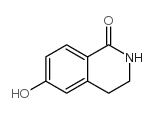 CAS#:22245-98-3
CAS#:22245-98-3 CAS#:1470-94-6
CAS#:1470-94-6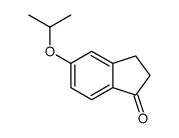 CAS#:760995-38-8
CAS#:760995-38-8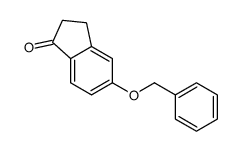 CAS#:78326-88-2
CAS#:78326-88-2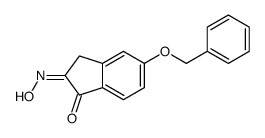 CAS#:88628-63-1
CAS#:88628-63-1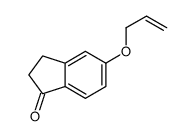 CAS#:37869-09-3
CAS#:37869-09-3 CAS#:38005-82-2
CAS#:38005-82-2
