Desferrichrome
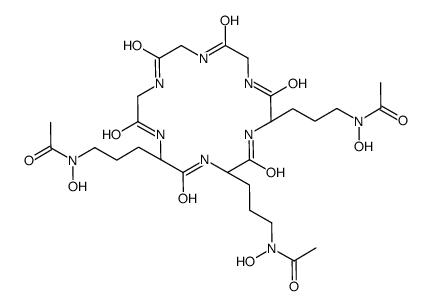
Desferrichrome structure
|
Common Name | Desferrichrome | ||
|---|---|---|---|---|
| CAS Number | 34787-28-5 | Molecular Weight | 687.69900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H45N9O12 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of DesferrichromeFerrichrome is a hydroxamate siderophore produced by various fungi, including U. sphaerogena, that facilitates iron chelation and uptake by these organisms. It can be used as a heterosiderophore by bacteria, including Pseudomonas aeruginosa and Vibrio parahaemolyticus. Ferrichrome (0.8 μM) inhibits concanavalin A-induced proliferation of mouse spleen monocytes and reduces the number of concanavalin A-stimulated CD4+ T cells expressing IL-2 receptor. It also inhibits the heme-catalyzed oxidation of LDL by hydrogen peroxide in a concentration-dependent manner. |
| Name | N-[3-[(2S,5S,8S)-5,8-bis[3-[acetyl(hydroxy)amino]propyl]-3,6,9,12,15,18-hexaoxo-1,4,7,10,13,16-hexazacyclooctadec-2-yl]propyl]-N-hydroxyacetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ferrichrome is a hydroxamate siderophore produced by various fungi, including U. sphaerogena, that facilitates iron chelation and uptake by these organisms. It can be used as a heterosiderophore by bacteria, including Pseudomonas aeruginosa and Vibrio parahaemolyticus. Ferrichrome (0.8 μM) inhibits concanavalin A-induced proliferation of mouse spleen monocytes and reduces the number of concanavalin A-stimulated CD4+ T cells expressing IL-2 receptor. It also inhibits the heme-catalyzed oxidation of LDL by hydrogen peroxide in a concentration-dependent manner. |
|---|---|
| Related Catalog |
| Molecular Formula | C27H45N9O12 |
|---|---|
| Molecular Weight | 687.69900 |
| Exact Mass | 687.31900 |
| PSA | 317.16000 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Microbial iron compounds.
Annu. Rev. Biochem. 50 , 715, (1981)
|
|
|
The biodistribution of 67Ga-labelled N-hydroxyamino acid derivatives in tumour-bearing rats.
Nucl. Med. Commun. 7(1) , 53-7, (1986) The biodistribution of 67Ga-labelled complexes of some mono- and di-N-hydroxyamino acid derivatives and of two siderophores (trihydroxamates) were evaluated in Rhabdomyosarcoma-bearing rats. It was fo... |
|
|
The solution conformations of ferrichrome and deferriferrichrome determined by 1H-NMR spectroscopy and computational modeling.
Biopolymers 30(3-4) , 239-56, (1990) We have applied computational procedures that utilize nmr data to model the solution conformation of ferrichrome, a rigid microbial iron transport cyclohexapeptide of known x-ray crystallographic stru... |
| Ferrichrome Iron-free |
| 4b8y |
| Desferrichrome |
| Desferriferrichrome |
| Deferriferrichrome |
| N-Dffc |
| N-Desferriferrichrome |
| Deferrichrome |
| Ferrichrome,deferri |