3-Aminobenzamide

3-Aminobenzamide structure
|
Common Name | 3-Aminobenzamide | ||
|---|---|---|---|---|
| CAS Number | 3544-24-9 | Molecular Weight | 136.151 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 329.6±25.0 °C at 760 mmHg | |
| Molecular Formula | C7H8N2O | Melting Point | 115-116 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 153.2±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3-Aminobenzamide3-Aminobenzamide is a potent inhibitor of PARP with IC50 of appr 50 nM in CHO cells, and acts as a mediator of oxidant-induced myocyte dysfunction during reperfusion. |
| Name | 3-aminobenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Aminobenzamide is a potent inhibitor of PARP with IC50 of appr 50 nM in CHO cells, and acts as a mediator of oxidant-induced myocyte dysfunction during reperfusion. |
|---|---|
| Related Catalog | |
| Target |
PARP:50 nM (IC50) |
| In Vitro | 3-Aminobenzamide (>1 μM) causes more than 95% inhibition of PARP activity without significant cellular toxicity. INO-1001 significantly sensitizes CHO cells by blocking most of the DNA repair occurring between radiation fractions[1]. 3-Aminobenzamide significantly improves endothelial function by enhancing the acetylcholine-induced, endothelium-dependent, nitric oxide mediated vasorelaxation after exposure with 400 μM H2O2[2]. |
| In Vivo | In a db/db (Leprdb/db) mouse model, 3-Aminobenzamide ameliorates diabetes-induced albumin excretion and mesangial expansion, and also decreases diabetes-induced podocyte depletion[3]. 3-Aminobenzamide (1.6 mg/kg via intracerebral injection) prevents NAD+ depletion and improves water maze performance after controlled cortical impact (CCI) in mice[4]. |
| Kinase Assay | PARP activity is measured with a PARP Activity Assay Kit. This method measures relative PARP activity by determining the level of incorporation of 3H-NAD into trichloroacetic acid (TCA) precipitable material in the presence of sheared genomic DNA, which activates PARP. The reaction mixture is added directly to washed cultures in 12-well culture plates and the reaction is allowed to proceed for 60 minutes at 37°C before the cells are removed mechanically, transferred to a microcentrifuge tube, and precipitated with ice-cold 5% TCA. |
| Animal Admin | Male db/db (Leprdb/db) mice, together with nondiabetic control db/m mice on C57BLKs/J background, are used. INO-1001 and PJ-34 treatment are initiated at 5 weeks of age. In sterile water that is sweetened with NutraSweet, 4.8 g/L 3-Aminobenzamide and 2.4 g/L PJ-34 is dissolved. Control animals receive sweetened water only. The average inhibitor consumption is 60 mg/kg 3-Aminobenzamide and 30 mg/kg PJ-34. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 329.6±25.0 °C at 760 mmHg |
| Melting Point | 115-116 °C(lit.) |
| Molecular Formula | C7H8N2O |
| Molecular Weight | 136.151 |
| Flash Point | 153.2±23.2 °C |
| Exact Mass | 136.063660 |
| PSA | 69.11000 |
| LogP | 0.33 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.633 |
| Water Solubility | ethanol: 50 mg/mL, clear, faintly yellow |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P280-P301 + P312 + P330-P304 + P340 + P312-P305 + P351 + P338-P337 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CU8992000 |
| HS Code | 2924299090 |
|
~94% 
3-Aminobenzamide CAS#:3544-24-9 |
| Literature: Rahaim Jr., Ronald J.; Maleczka Jr., Robert E. Synthesis, 2006 , # 19 p. 3316 - 3340 |
|
~99% 
3-Aminobenzamide CAS#:3544-24-9 |
| Literature: Brinchi, Lucia; Chiavini, Lisa; Goracci, Laura; Di Profio, Pietro; Germani, Raimondo Letters in Organic Chemistry, 2009 , vol. 6, # 2 p. 175 - 179 |
|
~91% 
3-Aminobenzamide CAS#:3544-24-9 |
| Literature: Sharghi, Hashem; Sarvari, Mona Hosseini Tetrahedron, 2002 , vol. 58, # 52 p. 10323 - 10328 |
|
~% 
3-Aminobenzamide CAS#:3544-24-9 |
| Literature: Bulletin of the Korean Chemical Society, , vol. 32, # 12 p. 4444 - 4446 |
|
~% 
3-Aminobenzamide CAS#:3544-24-9 |
| Literature: Journal of the American Pharmaceutical Association (1912-1977), , vol. 48, p. 201 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Protein poly(ADP-ribosyl)ation regulates arabidopsis immune gene expression and defense responses.
PLoS Genet. 11(1) , e1004936, (2015) Perception of microbe-associated molecular patterns (MAMPs) elicits transcriptional reprogramming in hosts and activates defense to pathogen attacks. The molecular mechanisms underlying plant pattern-... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
One-pot enzymatic conversion of carbon dioxide and utilization for improved microbial growth.
Environ. Sci. Technol. 49(7) , 4466-72, (2015) We developed a process for one-pot CO2 conversion and utilization based on simple conversion of CO2 to bicarbonate at ambient temperature with no energy input, by using the cross-linking-based composi... |
| meta-aminobenzoylamine |
| m-Aminobenzamide |
| 3-Aminobenzimide |
| Benzamide,m-amino |
| Benzamide, 3-amino- |
| 3-Aminobenzamide |
| 3-Amino-benzamide |
| MFCD00007989 |
| EINECS 222-586-9 |
| Benzamide,3-amino |
| INO1001 |
| meta-aminobenzamide |
| 3-NH2-Ph-CO-NH2 |
| 5-amino-benzamide |
| INO-1001 |
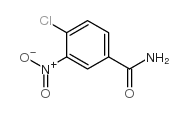
 CAS#:4403-70-7
CAS#:4403-70-7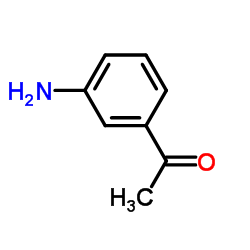 CAS#:99-03-6
CAS#:99-03-6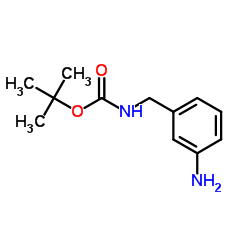 CAS#:147291-66-5
CAS#:147291-66-5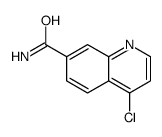 CAS#:178984-42-4
CAS#:178984-42-4 CAS#:122-49-6
CAS#:122-49-6 CAS#:74615-43-3
CAS#:74615-43-3![3-[(2-chloroacetyl)amino]benzamide structure](https://image.chemsrc.com/caspic/025/85126-66-5.png) CAS#:85126-66-5
CAS#:85126-66-5 CAS#:885274-73-7
CAS#:885274-73-7 CAS#:885278-66-0
CAS#:885278-66-0
