Piroxicam

Piroxicam structure
|
Common Name | Piroxicam | ||
|---|---|---|---|---|
| CAS Number | 36322-90-4 | Molecular Weight | 331.346 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 568.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C15H13N3O4S | Melting Point | 198-200°C | |
| MSDS | Chinese USA | Flash Point | 297.6±32.9 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PiroxicamPiroxicam is a non-steroidal anti-inflammatory drugs, acts as a COX inhibitor, with IC50s of 47, 25 μM for human monocyte COX-1 and COX-2, respectively. |
| Name | piroxicam |
|---|---|
| Synonym | More Synonyms |
| Description | Piroxicam is a non-steroidal anti-inflammatory drugs, acts as a COX inhibitor, with IC50s of 47, 25 μM for human monocyte COX-1 and COX-2, respectively. |
|---|---|
| Related Catalog | |
| Target |
COX-2:25 μM (IC50, in human monocytes) COX-1:47 μM (IC50, in human monocytes) |
| In Vitro | Piroxicam is a non-steroidal anti-inflammatory drugs, acts as a COX inhibitor, with IC50s of 47, 25 μM for human monocyte COX-1 and COX-2, respectively[1]. Piroxicam (167, 333, 500 μM) decreases cell population of T24 and the 5637 cells. Piroxicam (500 μM) also reduces the cell viability of T24 and 5637 cell, and is significantly effective when combined with 0.05 μM carboplatin. The combination also inhibits Ki-67 expression in booth cells[3]. |
| In Vivo | Piroxicam (0.3 mg/kg qd 24-h p.o.) reduces tumor volume in 12 of 18 dogs, and such and effect is via induction of apoptosis and reduction in urine basic fibroblast growth factor concentration[2]. |
| Cell Assay | Urinary bladder cancer cell lines are treated with graded concentrations of carboplatin (0.05, 0.5 and 1 μM) and Piroxicam (167, 333 and 500 μM) for 72 h to assess dose-response profiles. For the combination approach, 0.05 μM of carboplatin is used with 333 μM Piroxicam. Both drugs are freshly prepared before each experiment. An untreated control group (cells not exposed to carboplatin and Piroxicam) is used for all assays[3]. |
| Animal Admin | Dogs[2] Dogs undergo tumor staging, including thoracic and abdominal radiography, cystography, ultrasonography, and cystoscopy (with collection of tissue samples) before treatment and after 4 weeks of Piroxicam (0.3 mg/kg qd 24-h p. o.) treatment. Dogs receive no other cancer treatment during the 4 weeks of Piroxicam treatment. Tissue samples are immediately frozen in liquid nitrogen for PGE2 analysis or fixed in 10% neutral buffered formalin for immunohistochemical examination. Urine is also collected before and after Piroxicam treatment, aliquoted, and then stored at −80°C until analyzed[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 568.5±60.0 °C at 760 mmHg |
| Melting Point | 198-200°C |
| Molecular Formula | C15H13N3O4S |
| Molecular Weight | 331.346 |
| Flash Point | 297.6±32.9 °C |
| Exact Mass | 331.062683 |
| PSA | 107.98000 |
| LogP | 2.23 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36 |
| RIDADR | UN 2811 |
| RTECS | DL0705000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 3005101000 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Evaluation and enhancement of physical stability of semi-solid dispersions containing piroxicam into hard gelatin capsules.
Acta Pol. Pharm. 70(5) , 883-97, (2013) The aim of the study was to investigate the physical stability of the semi-solid dispersions into the hard gelatine capsules prepared with Gelucire 44/14, Labrasol and different additives such as micr... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide |
| 4-Hydroxy-2-methyl-N-pyridin-2-yl-2H-1,2-benzothiazin-3-carboxamid-1,1-dioxid |
| Riacen |
| 1,2-benzothiazine-3-carboxamide |
| Reudene |
| Zunden |
| Baxo |
| Roxiden |
| Larapam |
| Feldene |
| 4-hydroxy-2-méthyl-N-pyridin-2-yl-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxyde |
| Piroxicam |
| 4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide |
| 4-Hydroxy-2-methyl-N-2-pyridinyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide |
| Erazon |
| 4-Hydroxy-2-methyl-3-(pyrid-2-yl-carbamoyl)-2H-1,2-benzothiazine 1,1-dioxide |
| Artroxicam |
| Bruxicam |
| 4-Hydroxy-2-methyl-N-(2-pyridinyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide |
| 4-Hydroxy-2-methyl-3-(2-pyridylcarbamoyl)-2H-1,2-benzothiazine 1,1-Dioxide |
| Caliment |
| chf1251 |
| Improntal |
| Piroflex |
| MFCD00057317 |
| Geldene |
| Sasulen |
| 4-Hydroxy-2-methyl-N-(pyridin-2-yl)-2H-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide |
| Solocalm |
| EINECS 252-974-3 |
| 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-Dioxide |
| Flogobene |
| Pirkam |
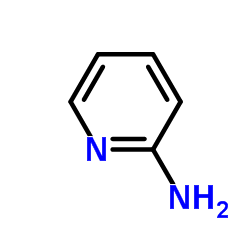 CAS#:504-29-0
CAS#:504-29-0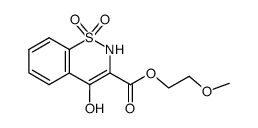 CAS#:80201-73-6
CAS#:80201-73-6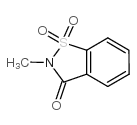 CAS#:15448-99-4
CAS#:15448-99-4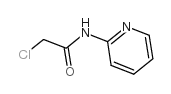 CAS#:5221-37-4
CAS#:5221-37-4 CAS#:35511-15-0
CAS#:35511-15-0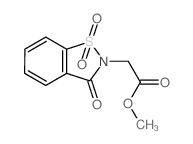 CAS#:6639-62-9
CAS#:6639-62-9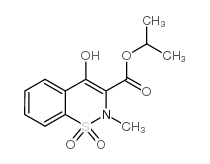 CAS#:118854-48-1
CAS#:118854-48-1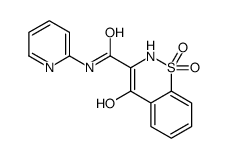 CAS#:65897-46-3
CAS#:65897-46-3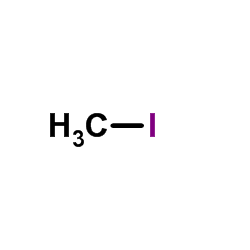 CAS#:74-88-4
CAS#:74-88-4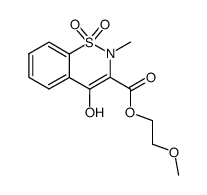 CAS#:80201-74-7
CAS#:80201-74-7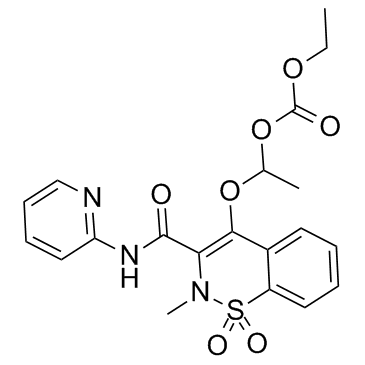 CAS#:99464-64-9
CAS#:99464-64-9![5-Methyl-3-(pyridin-2-yl)benzo[5,6][1,2]thiazino[3,4-e][1,3]oxazine-2,4(3H,5H)-dione 6,6-dioxide structure](https://image.chemsrc.com/caspic/471/90101-16-9.png) CAS#:90101-16-9
CAS#:90101-16-9
