Undecylenoyl chloride
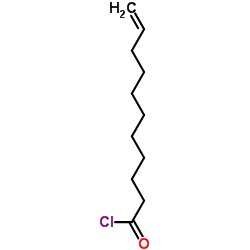
Undecylenoyl chloride structure
|
Common Name | Undecylenoyl chloride | ||
|---|---|---|---|---|
| CAS Number | 38460-95-6 | Molecular Weight | 202.721 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 252.2±0.0 °C at 760 mmHg | |
| Molecular Formula | C11H19ClO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 93.3±0.0 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | 10-Undecenoyl Chloride |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 252.2±0.0 °C at 760 mmHg |
| Molecular Formula | C11H19ClO |
| Molecular Weight | 202.721 |
| Flash Point | 93.3±0.0 °C |
| Exact Mass | 202.112442 |
| PSA | 17.07000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.452 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| RTECS | YQ2991000 |
| Packaging Group | III |
| Hazard Class | 8.0 |
| HS Code | 2916190090 |
|
~95% 
Undecylenoyl ch... CAS#:38460-95-6 |
| Literature: Balamurugan; Kannan Journal of Molecular Structure, 2009 , vol. 934, # 1-3 p. 44 - 52 |
|
~% 
Undecylenoyl ch... CAS#:38460-95-6 |
| Literature: Malanga, Corrado; Mannucci, Serena; Lardicci, Luciano Tetrahedron, 1998 , vol. 54, # 7 p. 1021 - 1028 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916190090 |
|---|---|
| Summary | 2916190090 unsaturated acyclic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:6.5%。general tariff:30.0% |
|
Photochemically modified diamond-like carbon surfaces for neural interfaces.
Mater. Sci. Eng. C. Mater. Biol. Appl. 58 , 1199-206, (2015) Diamond-like carbon (DLC) was modified using a UV functionalization method to introduce surface-bound amine and aldehyde groups. The functionalization process rendered the DLC more hydrophilic and sig... |
|
|
Olefin cross-metathesis as a source of polysaccharide derivatives: cellulose ω-carboxyalkanoates.
Biomacromolecules 15(1) , 177-87, (2014) Cross-metathesis has been shown for the first time to be a useful method for the synthesis of polysaccharide derivatives, focusing herein on preparation of cellulose ω-carboxyalkanoates. Commercially ... |
|
|
Preparation of cyclodextrin-modified monolithic hybrid columns for the fast enantioseparation of hydroxy acids in capillary liquid chromatography.
J. Sep. Sci. 39 , 1110-7, (2016) Cyclodextrins and their derivatives are one of the most common and successful chiral selectors. However, there have been few publications about the use of cyclodextrin-modified monoliths. In this stud... |
| undec-10-enoyl chloride |
| Undecylenoyl chloride |
| MFCD00000772 |
| 10-Undecenoyl chloride |
| EINECS 253-951-0 |
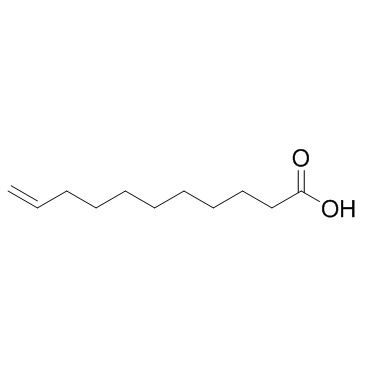

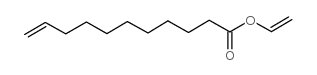 CAS#:5299-57-0
CAS#:5299-57-0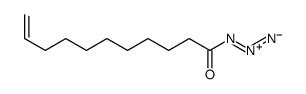 CAS#:143557-87-3
CAS#:143557-87-3 CAS#:141023-04-3
CAS#:141023-04-3 CAS#:506-13-8
CAS#:506-13-8 CAS#:17962-73-1
CAS#:17962-73-1 CAS#:28080-85-5
CAS#:28080-85-5 CAS#:29246-34-2
CAS#:29246-34-2 CAS#:29246-36-4
CAS#:29246-36-4 CAS#:24928-46-9
CAS#:24928-46-9
