Uridine diphosphate glucuronic acid ammonium
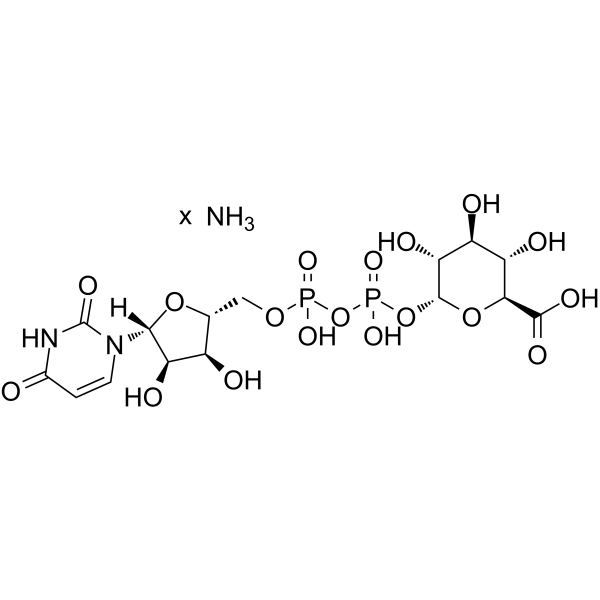
Uridine diphosphate glucuronic acid ammonium structure
|
Common Name | Uridine diphosphate glucuronic acid ammonium | ||
|---|---|---|---|---|
| CAS Number | 43195-60-4 | Molecular Weight | 597.31600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H25N3O18P2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Uridine diphosphate glucuronic acid ammoniumUridine diphosphate glucuronic acid (UDP-GlcA) ammonium is a cofactor that is formed by the catalytic activity of UDP-glucose dehydrogenase. Uridine diphosphate glucuronic acid (ammonium) is a central precursor in sugar nucleotide biosynthesis and common substrate for C4-epimerases and decarboxylases releasing UDP-galacturonic acid (UDP-GalA) and UDP-pentose products, respectively. Uridine diphosphate glucuronic acid (ammonium), as a glucuronic acid donor, can be used for for the research of the conjugation of bilirubin in the endoplasmic recticulum[1]. |
| Name | azane,(2S,3S,4S,5R,6R)-6-[[[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Uridine diphosphate glucuronic acid (UDP-GlcA) ammonium is a cofactor that is formed by the catalytic activity of UDP-glucose dehydrogenase. Uridine diphosphate glucuronic acid (ammonium) is a central precursor in sugar nucleotide biosynthesis and common substrate for C4-epimerases and decarboxylases releasing UDP-galacturonic acid (UDP-GalA) and UDP-pentose products, respectively. Uridine diphosphate glucuronic acid (ammonium), as a glucuronic acid donor, can be used for for the research of the conjugation of bilirubin in the endoplasmic recticulum[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Molecular Formula | C15H25N3O18P2 |
|---|---|
| Molecular Weight | 597.31600 |
| Exact Mass | 597.06100 |
| PSA | 337.18000 |
| Storage condition | 20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
|
Metabolic study of androsta-1,4,6-triene-3,17-dione in horses using liquid chromatography/high resolution mass spectrometry.
J. Steroid Biochem. Mol. Biol. 152 , 142-54, (2015) Androsta-1,4,6-triene-3,17-dione (ATD) is an irreversible steroidal aromatase inhibitor and is marketed as a supplement. It has been reported to effectively reduce estrogen biosynthesis and significan... |
|
|
Stable isotope labeling strategy for curcumin metabolite study in human liver microsomes by liquid chromatography-tandem mass spectrometry.
J. Am. Soc. Mass Spectrom. 26(4) , 686-94, (2015) The identification of drug metabolites is very important in drug development. Nowadays, the most widely used methods are isotopes and mass spectrometry. However, the commercial isotopic labeled reagen... |
|
|
Identification of diet-derived constituents as potent inhibitors of intestinal glucuronidation.
Drug Metab. Dispos. 42(10) , 1675-83, (2014) Drug-metabolizing enzymes within enterocytes constitute a key barrier to xenobiotic entry into the systemic circulation. Furanocoumarins in grapefruit juice are cornerstone examples of diet-derived xe... |
| UDP-GlcA |
| Uridine 5 inverted exclamation marka-diphosphoglucuronic acid ammonium salt |
| Uridine-diphosphate-glucuronic acid ammonium salt |