GW 627368
Modify Date: 2024-01-10 13:17:34
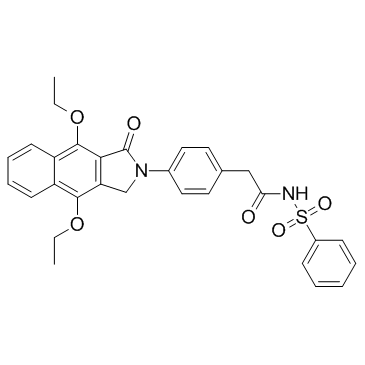
GW 627368 structure
|
Common Name | GW 627368 | ||
|---|---|---|---|---|
| CAS Number | 439288-66-1 | Molecular Weight | 544.618 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C30H28N2O6S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of GW 627368GW627368(GW627368X) is a novel, potent and selective competitive antagonist of prostanoid EP4 receptor(Ki= 100 nM) with additional human TP receptor affinity(Ki= 150 nM).IC50 value: Target: EP4 antagonistin vitro: At recombinant human prostanoid EP4 receptors expressed in HEK293 cells, GW627368X produced parallel rightward shifts of PGE2 concentration-effect (E/[A]) curves resulting in an affinity (pKb) estimate of 7.9 +/- 0.4. GW627368X appears to bind to human prostanoid TP receptors but not the TP receptors of other species. In human washed platelets, GW627368X (10 microM) produced 100% inhibition of U-46619 (EC100)-induced aggregation (approximate pA2 approximately 7.0) [1]. in vivo: Oral administration of GW627368X showed significant tumor regression characterized by tumor reduction and induction of apoptosis. Reduction in prostaglandin E2 synthesis also led to reduced level of VEGF in plasma [2]. |
| Name | N-(benzenesulfonyl)-2-[4-(4,9-diethoxy-3-oxo-1H-benzo[f]isoindol-2-yl)phenyl]acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | GW627368(GW627368X) is a novel, potent and selective competitive antagonist of prostanoid EP4 receptor(Ki= 100 nM) with additional human TP receptor affinity(Ki= 150 nM).IC50 value: Target: EP4 antagonistin vitro: At recombinant human prostanoid EP4 receptors expressed in HEK293 cells, GW627368X produced parallel rightward shifts of PGE2 concentration-effect (E/[A]) curves resulting in an affinity (pKb) estimate of 7.9 +/- 0.4. GW627368X appears to bind to human prostanoid TP receptors but not the TP receptors of other species. In human washed platelets, GW627368X (10 microM) produced 100% inhibition of U-46619 (EC100)-induced aggregation (approximate pA2 approximately 7.0) [1]. in vivo: Oral administration of GW627368X showed significant tumor regression characterized by tumor reduction and induction of apoptosis. Reduction in prostaglandin E2 synthesis also led to reduced level of VEGF in plasma [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C30H28N2O6S |
| Molecular Weight | 544.618 |
| Exact Mass | 544.166809 |
| PSA | 110.39000 |
| LogP | 4.95 |
| Index of Refraction | 1.645 |
| Storage condition | 2-8℃ |
| unii-a9p1zgy0se |
| 2-[4-(4,9-Diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]-N-(phenylsulfonyl)acetamide |
| Benzeneacetamide, 4-(4,9-diethoxy-1,3-dihydro-1-oxo-2H-benz[f]isoindol-2-yl)-N-(phenylsulfonyl)- |
| GW-627368X |
| GW627368 |