2-Aminopurine
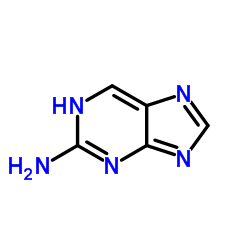
2-Aminopurine structure
|
Common Name | 2-Aminopurine | ||
|---|---|---|---|---|
| CAS Number | 452-06-2 | Molecular Weight | 135.127 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 328.2±25.0 °C at 760 mmHg | |
| Molecular Formula | C5H5N5 | Melting Point | 280-282 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 152.3±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Aminopurine2-Aminopurine, a fluorescent analog of guanosine and adenosine, is a widely used fluorescence-decay-based probe of DNA structure. When 2-Aminopurine is inserted in anoligonucleotide, its fluorescence is highly quenched by stacking with the natural bases. 2-Aminopurine has been used to probe nucleic acid structure and dynamics[1][2]. |
| Name | 2-aminopurine |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Aminopurine, a fluorescent analog of guanosine and adenosine, is a widely used fluorescence-decay-based probe of DNA structure. When 2-Aminopurine is inserted in anoligonucleotide, its fluorescence is highly quenched by stacking with the natural bases. 2-Aminopurine has been used to probe nucleic acid structure and dynamics[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Aminopurine (2AP) is not valuable as afluorescent label because its fluorescence is highly quenched by stacking with the natural bases, when it is inserted in anoligonucleotide. However, it is this very susceptibility to interbase quenching that makes 2AP an exquisitely sensitivefluorescent probe of nucleic acid structure[1]. 2-Aminopurine differs from adenine (6-aminopurine) only in the position of the exocyclic amine group, and yet its fluorescence intensity is one thousand times that of adenine[1]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 328.2±25.0 °C at 760 mmHg |
| Melting Point | 280-282 °C(lit.) |
| Molecular Formula | C5H5N5 |
| Molecular Weight | 135.127 |
| Flash Point | 152.3±23.2 °C |
| Exact Mass | 135.054489 |
| PSA | 80.48000 |
| LogP | -1.33 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.954 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UO7475000 |
| HS Code | 2933990090 |
|
~85% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Kusmierek, Jaroslaw; Czochralska, Barbara; Johansson, Nils Gunnar; Shugar, David Acta Chemica Scandinavica, Series B: Organic Chemistry and Biochemistry, 1987 , vol. 41, # 10 p. 701 - 707 |
|
~48% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Goto; Toya; Ohgi; Kondo Tetrahedron Letters, 1982 , vol. 23, # 12 p. 1271 - 1274 |
|
~90% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Kos, Nico J.; Plas, Henk C. van der Journal of Organic Chemistry, 1980 , vol. 45, # 15 p. 2942 - 2945 |
|
~% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Anufriev, M. A.; Ratsino, E. V.; Elagin, L. M.; Sokolov, L. B.; Komarov, E. V. J. Gen. Chem. USSR (Engl. Transl.), 1982 , vol. 52, # 5 p. 1015 - 1019,884 - 887 |
|
~% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Saladino, Raffaele; Botta, Giorgia; Delfino, Michela; Di Mauro, Ernesto Chemistry - A European Journal, 2013 , vol. 19, # 50 p. 16916 - 16922 |
|
~59% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Tisler, Miha; Stanovnik, Branko; Zrimsek, Zdenka Heterocycles, 1982 , vol. 17, p. 405 - 411 |
|
~% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Nair, Vasu; Buenger, Greg S. Journal of Organic Chemistry, 1990 , vol. 55, # 11 p. 3695 - 3697 |
|
~% 
2-Aminopurine CAS#:452-06-2 |
| Literature: Ratsep, Peter C.; Pless, Reynaldo C. Journal of Organic Chemistry, 1988 , vol. 53, # 14 p. 3241 - 3246 |
| Precursor 8 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Site-specific fluorescence dynamics in an RNA 'thermometer' reveals the role of ribosome binding in its temperature-sensitive switch function.
Nucleic Acids Res. 43(1) , 493-503, (2015) RNA thermometers control the translation of several heat shock and virulence genes by their temperature-sensitive structural transitions. Changes in the structure and dynamics of MiniROSE RNA, which r... |
|
|
Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-κB mediated and supports innate and adaptive anti-tumour immune priming.
Mol. Cancer 10 , 20, (2011) As well as inducing direct oncolysis, reovirus treatment of melanoma is associated with activation of innate and adaptive anti-tumour immune responses.Here we characterise the effects of conditioned m... |
|
|
Ligand-induced evolution of intrinsic fluorescence and catalytic activity from cobalt ferrite nanoparticles.
ChemPhysChem 16 , 1627-34, (2015) To develop CoFe(2)O(4) as magneto-fluorescent nanoparticles (NPs) for biomedical applications, it would be advantageous to identify any intrinsic fluorescence of this important magnetic material by si... |
| 2-Aminoperin |
| iso-Adenine |
| 2-Aminopurine |
| 3H-Purin-2-amine |
| MFCD07357244 |
| 7H-Purin-2-amine |
| 9H-Purin-2-amine |
| 2-amino purine |
| EINECS 207-197-4 |
| 9H-purin-2-ylamine |
| Purine, 2-amino- |
| 2-amino-6-purine |
| 1H-Purin-2-amine |
| 4-aminopurine |
| 2-amino-purin |
| SQ 22,451 |
![[(2S,5R)-5-(2-aminopurin-9-yl)oxolan-2-yl]methanol structure](https://image.chemsrc.com/caspic/016/107550-74-3.png)

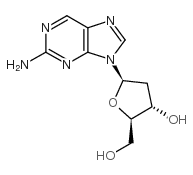
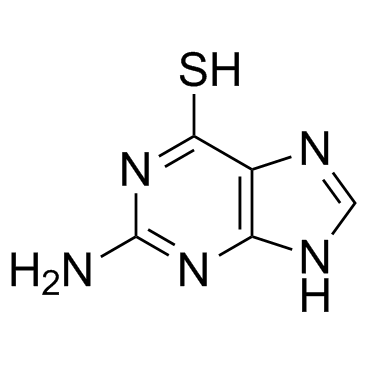
 CAS#:4546-54-7
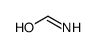
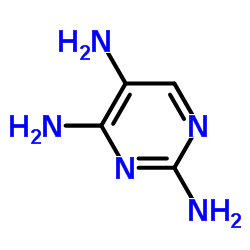
CAS#:4546-54-7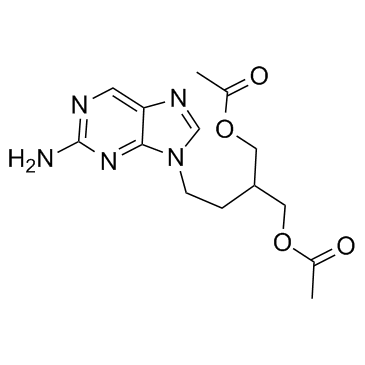 CAS#:104227-87-4
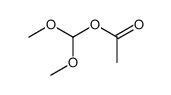
CAS#:104227-87-4![2-[(2-aminopurin-7-yl)methoxy]ethanol structure](https://image.chemsrc.com/caspic/418/114810-83-2.png) CAS#:114810-83-2
CAS#:114810-83-2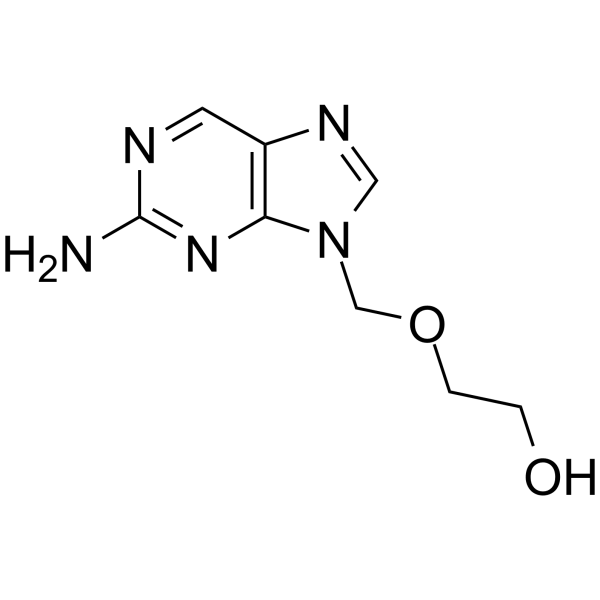 CAS#:84408-37-7
CAS#:84408-37-7
