AEE788 (NVP-AEE788)
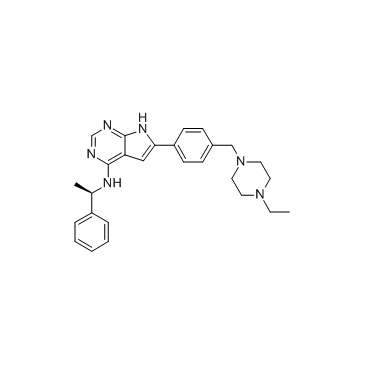
AEE788 (NVP-AEE788) structure
|
Common Name | AEE788 (NVP-AEE788) | ||
|---|---|---|---|---|
| CAS Number | 497839-62-0 | Molecular Weight | 440.583 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C27H32N6 | Melting Point | 247 °C | |
| MSDS | USA | Flash Point | N/A | |
Use of AEE788 (NVP-AEE788)AEE788 is an inhibitor of the EGFR and ErbB2 with IC50 values of 2 and 6 nM, respectively. |
| Name | 6-(4-((4-ethyl-1-piperazinyl)methyl)phenyl)-N-((1R)-1-phenylethyl)-1H-Pyrrolo(2,3-D)pyrimidin-4-amine |
|---|---|
| Synonym | More Synonyms |
| Description | AEE788 is an inhibitor of the EGFR and ErbB2 with IC50 values of 2 and 6 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
EGFR:2 nM (IC50) ErbB2:6 nM (IC50) |
| In Vitro | AEE788 inhibits EGFR and VEGF receptor tyrosine kinases in the nM range (IC50:EGFR 2 nm, ErbB2 6 nm, KDR 77 nm, and Flt-1 59 nm). In cells, growth factor-induced EGFR and ErbB2 phosphorylation is also efficiently inhibited (IC50:11 and 220 nm, respectively). AEE788 demonstrates antiproliferative activity against a range of EGFR and ErbB2-overexpressing cell lines (including EGFRvIII-dependent lines) and inhibits the proliferation of epidermal growth factor- and VEGF-stimulated human umbilical vein endothelial cells[1]. Treatment of cutaneous SCC cells with AEE788 leads to dose-dependent inhibition of EGFR and VEGFR-2 phosphorylation, growth inhibition, and induction of apoptosis[2]. |
| In Vivo | AEE788 efficiently inhibits growth factor-induced EGFR and ErbB2 phosphorylation in tumors for >72 h. AEE788 also inhibits VEGF-induced angiogenesis in a murine implant model[1]. In mice treated with AEE788, tumor growth is inhibited by 54% at 21 days after the start of treatment compared with control mice[2]. |
| Kinase Assay | The invitro kinase assays are performed in 96-well plates (30 μL) at ambient temperature for 15–45 min using the recombinant glutathione S-transferase-fused kinase domains (4–100 ng, depending on specific activity). [γ33P]ATP is used as phosphate donor and polyGluTyr-(4:1) peptide as acceptor. Assays are optimized for each kinase using the following ATP concentrations: 1.0 μM (c-Kit, c-Met, c-Fms, c-Raf-1, and RET), 2.0 μM (EGFR, ErbB2, ErbB3, and ErbB4), 5.0 μM (c-Abl), 8.0 μM (Flt-1, Flt-3, Flt-4, Flk, KDR, FGFR-1, and Tek), 10.0 μM (PDGF receptor-β, protein kinase C-α, and cyclin-dependent kinase 1), and 20.0 μM (c-Src and protein kinase A). The reaction is terminated by the addition of 20 μL 125 mM EDTA. Thirty μL (c-Abl, c-Src, insulin-like growth factor-1R, RET-Men2A, and RET-Men2B) or 40 μL (all other kinases) of the reaction mixture is transferred onto Immobilon-polyvinylidene difluoride membrane, presoaked with 0.5% H3PO4 and mounted on a vacuum manifold. Vacuum is then applied and each well rinsed with 200 μL 0.5% H3PO4. Membranes are removed and washed four times. Dried membranes are counted. IC50 are calculated by linear regression analysis of the percentage inhibition and are averages of at least three determinations[1]. |
| Cell Assay | AEE788 is dissolved in 90% polyethylene glycol 300 plus 10% 1-methyl-2-pyrrolidinone to a concentration of 6.25 mg/mL. Tumor cells are seeded into 96-well plates in complete medium and allowed to attach for 24 hours. The cultures are re-fed with medium with 2% serum. After 24 hours, cells are treated with different concentrations (0-2 μM) of AEE788 (negative control with DMSO alone) for 72 hours. After a 2-hour incubation in medium containing 0.42 mg/mL MTT, the cells are lysed in 100 μL DMSO. The conversion of MTT to formazan is measured at an absorbance of 570 nm[2] |
| Animal Admin | Mice: AEE788 is diluted in DMSO and diluted in the optimal medium. BALB/c mice bearing s.c. A-431 squamous tumors (3 animals/group) or HC11-NeuT-driven breast tumors (2 animals/group) are dosed orally with 30 mg/kg of AEE788 or vehicle once daily for 5 days. At different time points after the end of compound treatment and before sacrificing the animals the mice are given i.v. 500 μg EGF/kg body weight or 0.2 ml 0.9% w/v NaCl as vehicle control. Five min after EGF administration, the mice are sacrificed, tumors are removed[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 247 °C |
| Molecular Formula | C27H32N6 |
| Molecular Weight | 440.583 |
| Exact Mass | 440.268860 |
| PSA | 63.31000 |
| LogP | 4.76 |
| Index of Refraction | 1.665 |
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | UY8836200 |
|
~% 
AEE788 (NVP-AEE788) CAS#:497839-62-0 |
| Literature: WO2007/17468 A2, ; Page/Page column 7-8 ; |
|
~% 
AEE788 (NVP-AEE788) CAS#:497839-62-0 |
| Literature: WO2007/17468 A2, ; Page/Page column 7-8 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| Furanace-10 |
| Furpyrinol |
| Furanace |
| (R)-6-(4-((4-Ethylpiperazin-1-yl)methyl)phenyl)-N-(1-phenylethyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine |
| Nifurpirinol |
| 7H-Pyrrolo[2,3-d]pyrimidin-4-amine, 6-[4-[(4-ethyl-1-piperazinyl)methyl]phenyl]-N-[(1R)-1-phenylethyl]- |
| {6-[2-(5-nitro-furan-2-yl)-vinyl]-pyridin-2-yl}-methanol |
| Nifurpirinolum [INN-Latin] |
| AEE788 |
| NVP-AEE788 |
| [6-[4-(4-ethyl-piperazine-1-ylmethyl)-phenyl]-7H-pyrrolo[2,3-d]pyrinidinpyrimidin-4-yl]-((R)-1-phenyl-ethyl)-amine |
| Nifurpirinol [USAN:INN] |
| Furpirinol |
| 6-{4-[(4-Ethyl-1-piperazinyl)methyl]phenyl}-N-[(1R)-1-phenylethyl]-1H-pyrrolo[2,3-d]pyrimidin-4-amine |
| [6-[4-[(4-ethylpiperazine-1-yl)methyl]phenyl]-7H-pyrrolo[2,3-d]pyrimidine-4-yl]-((R)-1-phenylethyl)amine |
| {6-[4-(4-ethyl-piperazin-1-ylmethyl)-phenyl]-7H-pyrrolo[2.3-d]-pyrimidin-4-yl}-((R)-1-phenyl-ethyl)-amine |

![Ethyl 4-(4-{[(1R)-1-phenylethyl]amino}-1H-pyrrolo[2,3-d]pyrimidin-6-yl)benzoate structure](https://image.chemsrc.com/caspic/135/497841-26-6.png)