H-Tyr-NH2
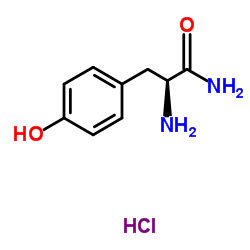
H-Tyr-NH2 structure
|
Common Name | H-Tyr-NH2 | ||
|---|---|---|---|---|
| CAS Number | 4985-46-0 | Molecular Weight | 216.665 | |
| Density | 1.268g/cm3 | Boiling Point | 437.7ºC at 760mmHg | |
| Molecular Formula | C9H13ClN2O2 | Melting Point | 153.5°C | |
| MSDS | Chinese USA | Flash Point | 218.5ºC | |
| Name | L-tyrosinamide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.268g/cm3 |
|---|---|
| Boiling Point | 437.7ºC at 760mmHg |
| Melting Point | 153.5°C |
| Molecular Formula | C9H13ClN2O2 |
| Molecular Weight | 216.665 |
| Flash Point | 218.5ºC |
| Exact Mass | 216.066559 |
| PSA | 89.34000 |
| LogP | 1.94990 |
| Index of Refraction | 1.612 |
| Storage condition | -15°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Solvent selection and optimization of α-chymotrypsin-catalyzed synthesis of N-Ac-Phe-Tyr-NH2 using mixture design and response surface methodology.
Biotechnol. Prog. 28(6) , 1443-9, (2012) A peptide, N-Ac-Phe-Tyr-NH(2) , with angiotensin I-converting enzyme (ACE) inhibitor activity was synthesized by an α-chymotrypsin-catalyzed condensation reaction of N-acetyl phenylalanine ethyl ester... |
|
|
Enzymatic copolymerization alters the structure of unpolymerized mixtures of the biomimetic monomers: the amphiphilic decyl ester of L-tyrosine and L-tyrosineamide--an AFM investigation of nano- to micrometer-scale structure differences.
Biomacromolecules 5(5) , 1869-76, (2004) Previously, we have shown that the amphiphilic decyl esters of both D- and L-tyrosine (DELT) self-assemble in aqueous solution above their critical micelle concentration values to form long rodlike st... |
|
|
Derivatization and purification of bisecting tyrosinamide-oligosaccharides from ovotransferrin.
Arch. Biochem. Biophys. 315(2) , 460-6, (1994) The major N-linked oligosaccharides from ovotransferrin were purified on a large scale. The oligosaccharides were released from 5 g of the glycoprotein using N-glycosidase F and isolated from a mixed ... |
| Benzenepropanamide, α-amino-4-hydroxy-, (αS)-, hydrochloride (1:1) |
| (2S)-2-amino-3-(4-hydroxyphenyl)propanamide |
| L-Tyrosinamide hydrochloride (1:1) |
| (S)-2-Amino-3-(4-hydroxyphenyl)propanamide |
| H-Tyr-NH2 |
 CAS#:1080-06-4
CAS#:1080-06-4 CAS#:60-18-4
CAS#:60-18-4 CAS#:3417-91-2
CAS#:3417-91-2 CAS#:34081-17-9
CAS#:34081-17-9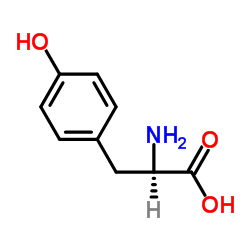 CAS#:556-02-5
CAS#:556-02-5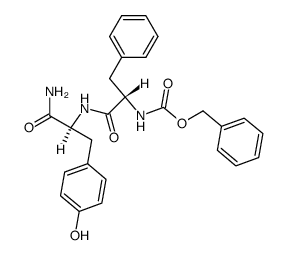 CAS#:57851-59-9
CAS#:57851-59-9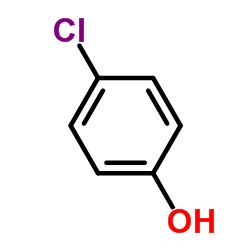 CAS#:106-48-9
CAS#:106-48-9 CAS#:14191-95-8
CAS#:14191-95-8
