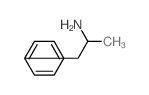
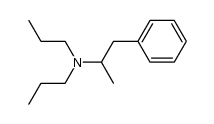
1-phenyl-N-propylpropan-2-amine
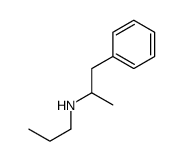
1-phenyl-N-propylpropan-2-amine structure
|
Common Name | 1-phenyl-N-propylpropan-2-amine | ||
|---|---|---|---|---|
| CAS Number | 51799-32-7 | Molecular Weight | 177.28600 | |
| Density | 0.897g/cm3 | Boiling Point | 254.4ºC at 760mmHg | |
| Molecular Formula | C12H19N | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 103.6ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
| Name | 1-phenyl-N-propylpropan-2-amine |
|---|---|
| Synonym | More Synonyms |
| Density | 0.897g/cm3 |
|---|---|
| Boiling Point | 254.4ºC at 760mmHg |
| Molecular Formula | C12H19N |
| Molecular Weight | 177.28600 |
| Flash Point | 103.6ºC |
| Exact Mass | 177.15200 |
| PSA | 12.03000 |
| LogP | 3.00810 |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| HS Code | 2921499090 |
|
~% 
1-phenyl-N-prop... CAS#:51799-32-7 |
| Literature: Jacobsen et al. Skand.Arch.Physiol., 1938 , vol. 79, p. 258,279 |
|
~% 
1-phenyl-N-prop... CAS#:51799-32-7 |
| Literature: Kanao Yakugaku Zasshi, 1930 , vol. 50, p. 338,343;dtsch.Ref.S.43,46 Chem. Zentralbl., 1930 , vol. 101, # II p. 1695 |
|
~% 
1-phenyl-N-prop... CAS#:51799-32-7 |
| Literature: Testa; Salvesen Journal of Pharmaceutical Sciences, 1980 , vol. 69, # 5 p. 497 - 501 |
|
~% 
1-phenyl-N-prop... CAS#:51799-32-7 |
| Literature: Testa; Salvesen Journal of Pharmaceutical Sciences, 1980 , vol. 69, # 5 p. 497 - 501 |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
A convenient derivatization method for gas chromatography/mass spectrometric determination of phenmetrazine in urine using 2,2,2-trichloroethyl chloroformate.
J. Forensic Sci. 43(3) , 630-5, (1998) Phenmetrazine is a central nervous system stimulant currently used as an anorectic agent. The drug is abused and is reported to cause death from overdose. We describe a new derivatization method for p... |
|
|
Amphetamine in rat brain after intraperitoneal injection of N-alkylated analogues.
Prog. Neuropsychopharmacol. Biol. Psychiatry 7(4-6) , 813-6, (1983) Three N-alkylated analogues of amphetamine were administered intraperitoneally to male Sprague-Dawley rats and whole brain levels of amphetamine (AM) and the N-alkyl analogue were determined one hour ... |
|
|
In vivo and in vitro o-methylation of 1-(3,4-dihydroxyphenyl)-2-(n-propyl-amino) propane - an intermediate in N-(N-propyl) amphetamine metabolism.
Res. Commun. Chem. Pathol. Pharmacol. 36(1) , 173-6, (1982) In vitro metabolism of 1-(3,4-dihydroxyphenyl)-2-(n-propylamino)-propane (Id) by rat liver cytosol in the presence of S-adenosyl-L-methyl-methionine produced almost quantitatively the corresponding 3-... |
| 1-Phenyl-2-n-propylamino-propan [German] |
| 1-Phenyl-2-n-propylamino-propane |
| 1-Phenyl-2-propylaminopropane |
| d-N-Propylamphetamine |