H-Ala-Obzl.HCl
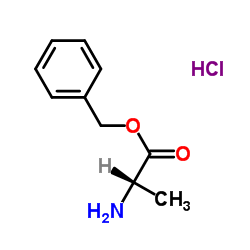
H-Ala-Obzl.HCl structure
|
Common Name | H-Ala-Obzl.HCl | ||
|---|---|---|---|---|
| CAS Number | 5557-83-5 | Molecular Weight | 215.677 | |
| Density | 1.1g/cm3 | Boiling Point | 293ºC at 760 mmHg | |
| Molecular Formula | C10H14ClNO2 | Melting Point | 135 to 145ºC | |
| MSDS | Chinese USA | Flash Point | 131ºC | |
| Name | L-Alanine Benzyl Ester Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1g/cm3 |
|---|---|
| Boiling Point | 293ºC at 760 mmHg |
| Melting Point | 135 to 145ºC |
| Molecular Formula | C10H14ClNO2 |
| Molecular Weight | 215.677 |
| Flash Point | 131ºC |
| Exact Mass | 215.071304 |
| PSA | 52.32000 |
| LogP | 2.57930 |
| Index of Refraction | 1.53 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2922499990 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
The Benzyl Ester Group of Amino Acid Monomers Enhances Substrate Affinity and Broadens the Substrate Specificity of the Enzyme Catalyst in Chemoenzymatic Copolymerization.
Biomacromolecules 17 , 314-23, (2016) The chemoenzymatic polymerization of amino acid monomers by proteases involves a two-step reaction: the formation of a covalent acyl-intermediate complex between the protease and the carboxyl ester gr... |
|
|
Positively charged microemulsions for topical application.
Int. J. Pharm. 346 , 119-123, (2008) The study reports pig-skin permeation and skin accumulation of miconazole nitrate (MCZ) from positively charged microemulsions containing water, 1-decanol/1-dodecanol (2:1, w/w), lecithin and/or decyl... |
|
|
Synthesis, QSAR and anti-HIV activity of new 5-benzylthio-1,3,4-oxadiazoles derived from α-amino acids.
J. Enzyme Inhib. Med. Chem. 26 , 668-680, (2011) 2-(1-[(4-Chloro/methylphenylsulfonylamino)alkyl]-5-thioxo-4,5-dihydro-1,3,4-oxadiazoles (4a-e) were synthesized, in four steps, via the sulfonyl derivatives of l-amino acids (l-alanine, l-methionine a... |
| L-Alanine benzyl ester hydrochloride |
| EINECS 226-920-4 |
| MFCD00054340 |
| Alanine, phenylmethyl ester, hydrochloride (1:1) |
| Benzyl alaninate hydrochloride (1:1) |
| L-Alaninebenzylesterhydrochloride |

