s(+)-3 4-mdma hcl
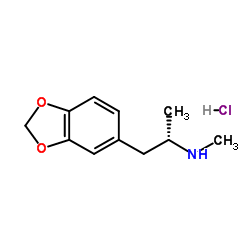
s(+)-3 4-mdma hcl structure
|
Common Name | s(+)-3 4-mdma hcl | ||
|---|---|---|---|---|
| CAS Number | 64057-70-1 | Molecular Weight | 229.703 | |
| Density | N/A | Boiling Point | 100-110ºC at 0.4 mmHg | |
| Molecular Formula | C11H16ClNO2 | Melting Point | 147-148° (Bailey); mp 152-153° (Braun) | |
| MSDS | Chinese USA | Flash Point | 11ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
| Name | 3,4-Methylenedioxy Methamphetamine Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 100-110ºC at 0.4 mmHg |
|---|---|
| Melting Point | 147-148° (Bailey); mp 152-153° (Braun) |
| Molecular Formula | C11H16ClNO2 |
| Molecular Weight | 229.703 |
| Flash Point | 11ºC |
| Exact Mass | 229.086960 |
| PSA | 30.49000 |
| LogP | 2.75860 |
| Appearance of Characters | solid | white |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | P261-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T+ |
| Risk Phrases | 26/27/28-39/23/24/25-23/24/25-11 |
| Safety Phrases | 22-36/37/39-45-36/37-16-7-26 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | SH5700000 |
|
Measuring effects of MDMA (ecstasy) abuse on the rate of cerebral serotonin synthesis.
J. Neurochem. 131(5) , 541-5, (2014)
|
|
|
Brain serotonin synthesis in MDMA (ecstasy) polydrug users: an alpha-[(11) C]methyl-l-tryptophan study.
J. Neurochem. 131(5) , 634-44, (2014) 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) use may have long-term neurotoxic effects. In this study, positron emission tomography with the tracer alpha-[(11) C]methyl-l-tryptophan ((11) C-AMT) ... |
|
|
3,4-Methylenedioxy-methamphetamine induces in vivo regional up-regulation of central nicotinic receptors in rats and potentiates the regulatory effects of nicotine on these receptors.
Neurotoxicology 35 , 41-9, (2013) Nicotine (NIC), the main psychostimulant compound of smoked tobacco, exerts its effects through activation of central nicotinic acetylcholine receptors (nAChR), which become up-regulated after chronic... |
| (2S)-1-(1,3-Benzodioxol-5-yl)-N-methyl-2-propanamine hydrochloride (1:1) |
| 1,3-Benzodioxole-5-ethanamine, N,α-dimethyl-, (αS)-, hydrochloride (1:1) |
| 1-(1,3-benzodioxol-5-yl)-N-methylpropan-2-amine,hydrochloride |