Quinoline-3-carboxylic acid
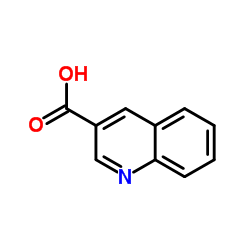
Quinoline-3-carboxylic acid structure
|
Common Name | Quinoline-3-carboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 6480-68-8 | Molecular Weight | 173.168 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 348.7±17.0 °C at 760 mmHg | |
| Molecular Formula | C10H7NO2 | Melting Point | 277-280 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 164.7±20.9 °C | |
| Name | 3-Quinolinecarboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.7±17.0 °C at 760 mmHg |
| Melting Point | 277-280 °C(lit.) |
| Molecular Formula | C10H7NO2 |
| Molecular Weight | 173.168 |
| Flash Point | 164.7±20.9 °C |
| Exact Mass | 173.047684 |
| PSA | 50.19000 |
| LogP | 2.18 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.685 |
| Storage condition | Refrigerator |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933499090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Effects of norfloxacin on hepatic genes expression of P450 isoforms (CYP1A and CYP3A), GST and P-glycoprotein (P-gp) in Swordtail fish (Xiphophorus Helleri).
Ecotoxicology 24 , 1566-73, (2015) The presence of antibiotics including norfloxacin in the aquatic environment may cause adverse effects in non-target organisms. But the toxic mechanisms of fluoroquinolone to fish species are still no... |
|
|
Small-molecule inhibitors of histone acetyltransferase activity: identification and biological properties.
J. Med. Chem. 49 , 6897-907, (2006) Starting from a yeast phenotypic screening performed on 21 compounds, we described the identification of two small molecules (9 and 18) able to significantly reduce the S. cerevisiae cell growth, thus... |
|
|
Novel amino-substituted 3-quinolinecarboxylic acid antibacterial agents: synthesis and structure-activity relationships.
J. Med. Chem. 27(9) , 1103-8, (1984) A series of novel 3-quinolinecarboxylic acid derivatives have been prepared and their antibacterial activity evaluated. These derivatives are characterized by fluorine attached to the 6-position and s... |
| EINECS 229-337-3 |
| MFCD00006770 |
| 3-Quinolinecarboxylic acid |
| Quinoline-3-carboxylic acid |
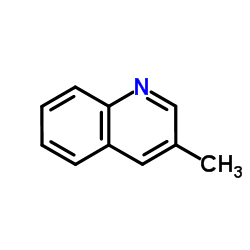 CAS#:612-58-8
CAS#:612-58-8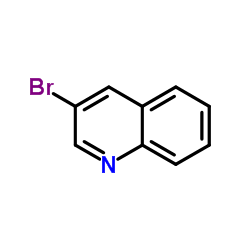 CAS#:5332-24-1
CAS#:5332-24-1 CAS#:201230-82-2
CAS#:201230-82-2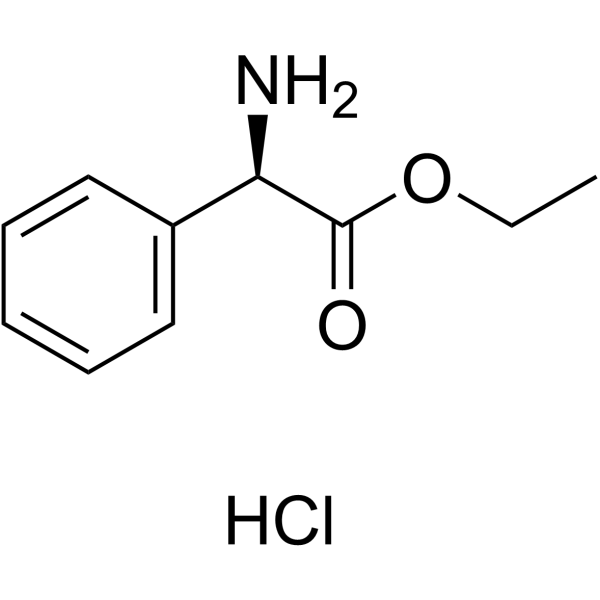 CAS#:17609-48-2
CAS#:17609-48-2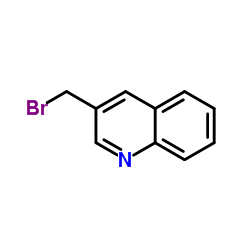 CAS#:120277-70-5
CAS#:120277-70-5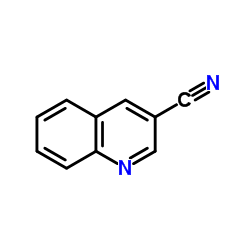 CAS#:34846-64-5
CAS#:34846-64-5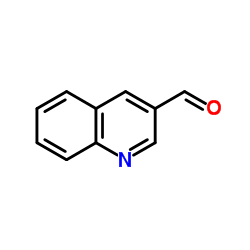 CAS#:13669-42-6
CAS#:13669-42-6 CAS#:124-38-9
CAS#:124-38-9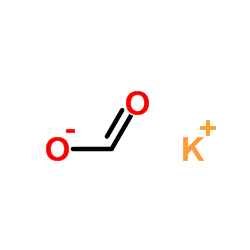 CAS#:590-29-4
CAS#:590-29-4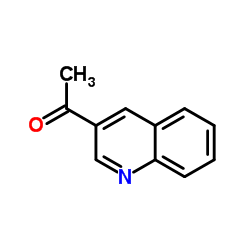 CAS#:33021-53-3
CAS#:33021-53-3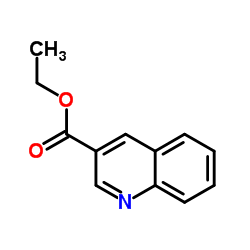 CAS#:50741-46-3
CAS#:50741-46-3 CAS#:491-34-9
CAS#:491-34-9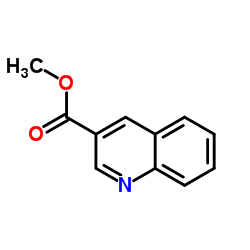 CAS#:53951-84-1
CAS#:53951-84-1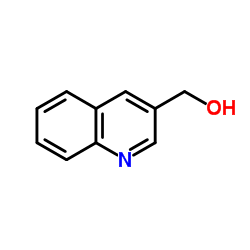 CAS#:13669-51-7
CAS#:13669-51-7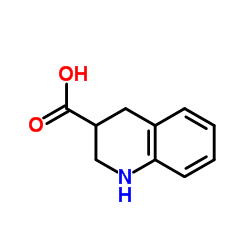 CAS#:114527-53-6
CAS#:114527-53-6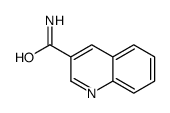 CAS#:6480-67-7
CAS#:6480-67-7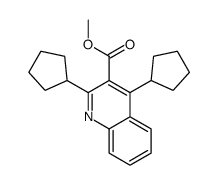 CAS#:753487-72-8
CAS#:753487-72-8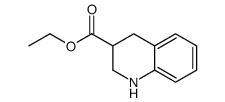 CAS#:67752-37-8
CAS#:67752-37-8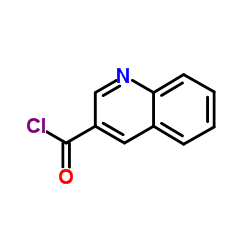 CAS#:84741-86-6
CAS#:84741-86-6
